(UroToday.com) The 2024 ESMO annual meeting included a session on prostate cancer, featuring a presentation by Dr. Fred Saad discussing the efficacy and safety of darolutamide plus ADT in patients with mHSPC from the phase 3 ARANOTE trial. In ARASENS, darolutamide + ADT + docetaxel significantly improved overall survival versus ADT + docetaxel in patients with mHSPC, and incidences of treatment-emergent adverse events were similar in both groups.1 The phase 3 global ARANOTE trial (NCT04736199) compared darolutamide + ADT versus ADT in patients with mHSPC. At ESMO 2024, Dr. Saad presented the primary results of the trial.
Eligible patients had mHSPC by conventional imaging, an ECOG performance status of 0–2, and started ADT ≤ 12 weeks. Patients were randomized 2:1 to darolutamide 600 mg twice daily or placebo, each with ADT. The primary endpoint was radiological progression-free survival, and secondary endpoints included overall survival, time to initiation of subsequent anticancer therapy, time to castration-resistant prostate cancer (CRPC), time to PSA progression, time to pain progression, and safety. The trial design for ARANOTE is below:
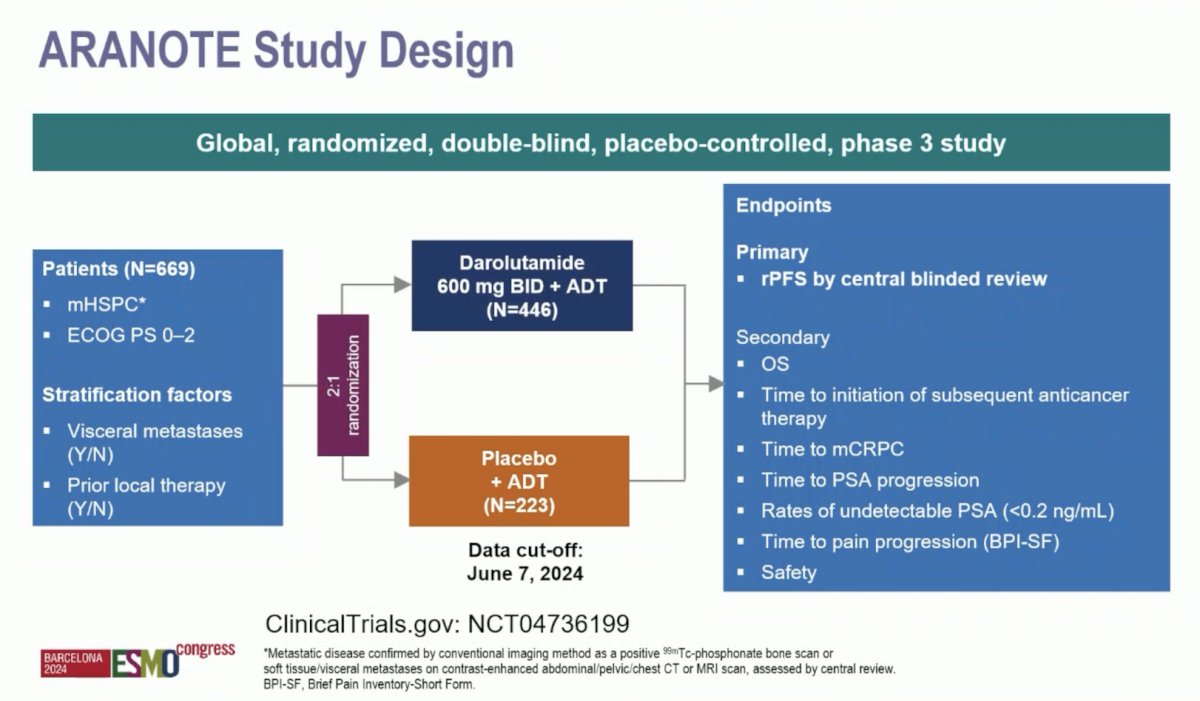
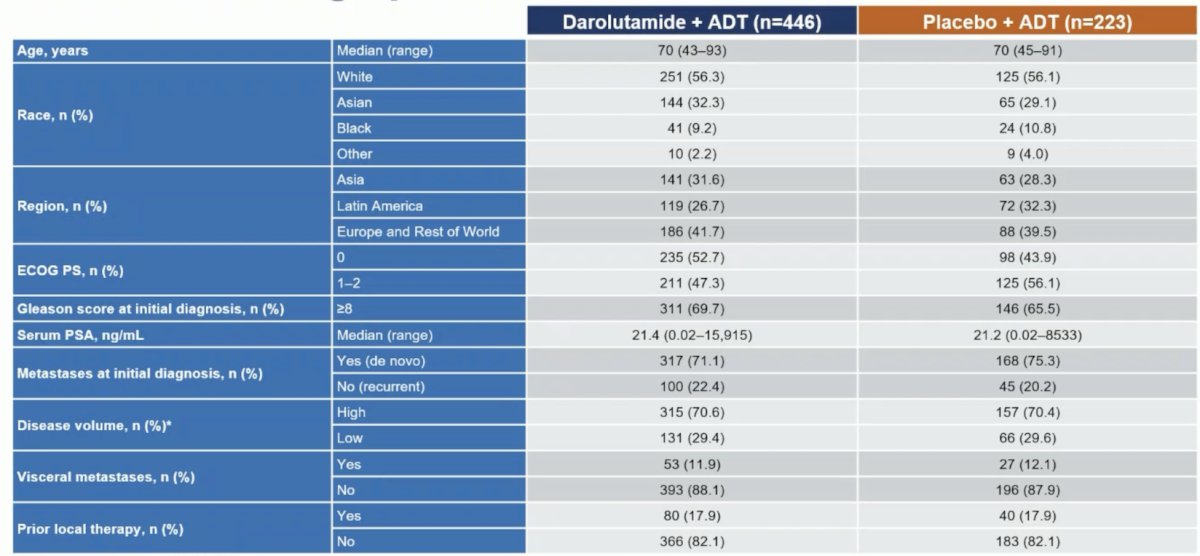
A total of 669 patients were randomized (darolutamide, n = 446; placebo, n = 223). The median age was 70 years, 31% were Asian, 9.7% were Black, the median PSA at baseline was 21.3 ng/mL, and 71% had high-volume mHSPC:
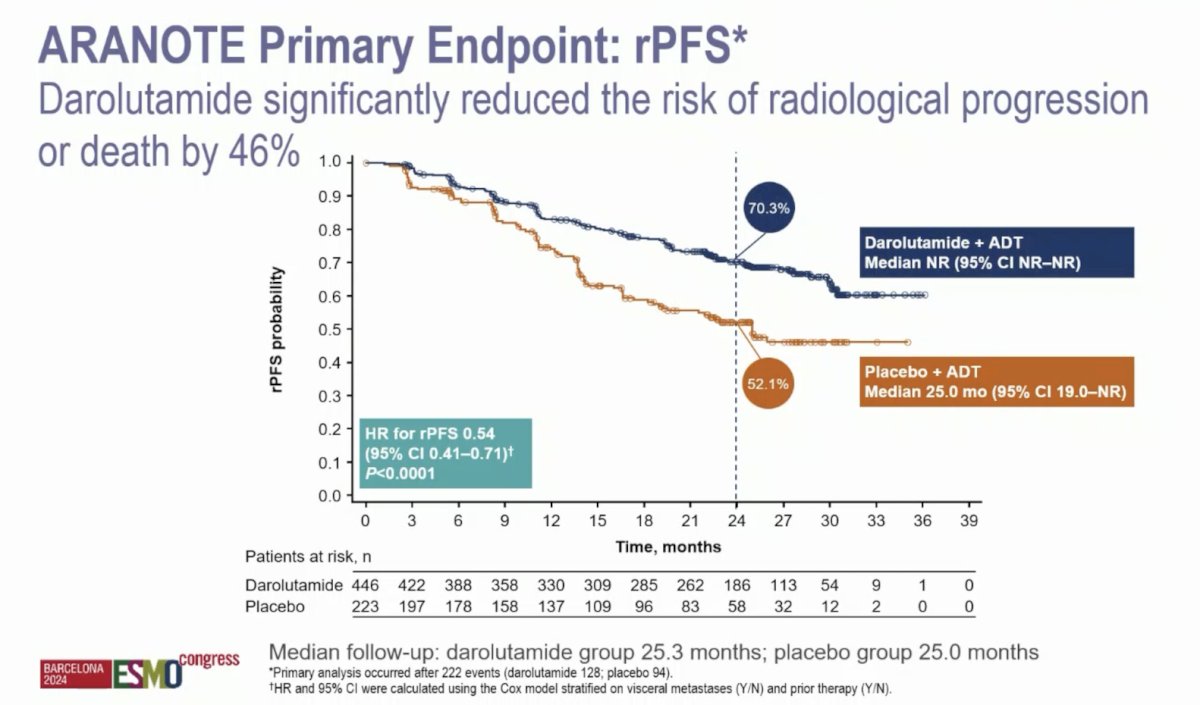
At the primary data cutoff of June 7, 2024, darolutamide + ADT significantly improved radiological progression-free survival versus placebo + ADT (HR 0.54, 95% CI 0.41–0.71):
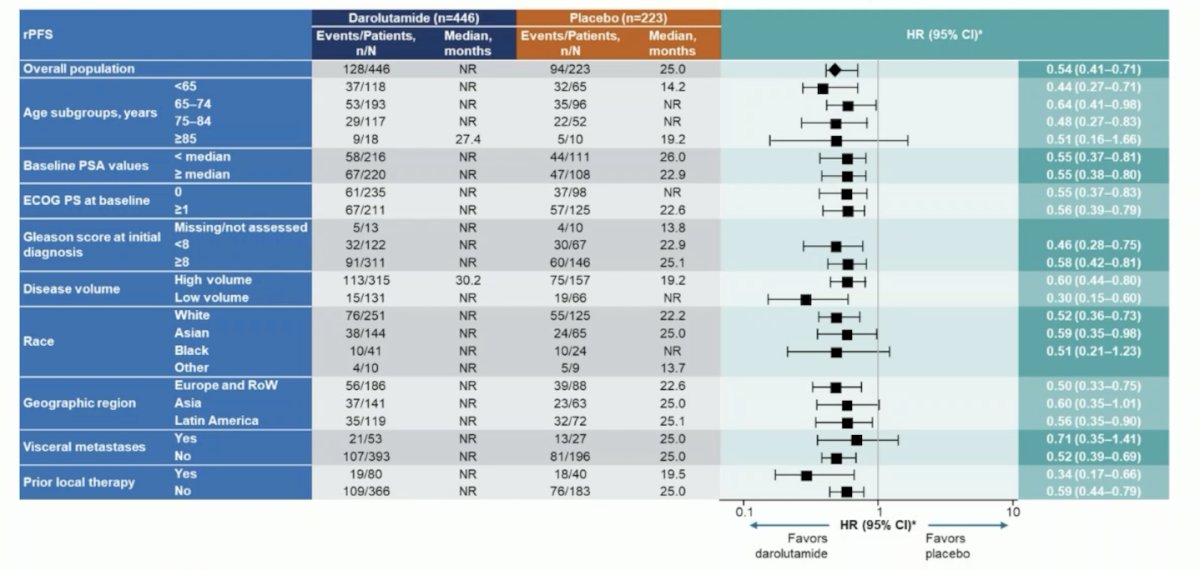
There were also consistent benefits observed across prespecified subgroups, including patients with high- and low-volume mHSPC:
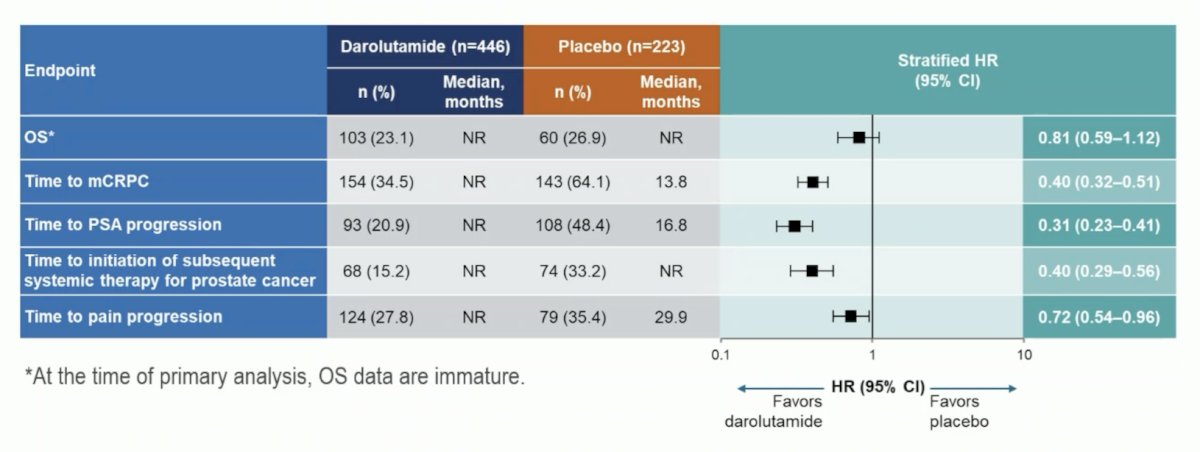
Darolutamide was associated with a positive trend for overall survival (HR 0.81, 95% CI 0.59–1.12) and clinical benefits across all secondary efficacy endpoints, including time to CRPC (HR 0.40, 95% CI 0.32–0.51) and time to pain progression (HR 0.72, 95% CI 0.54–0.96):
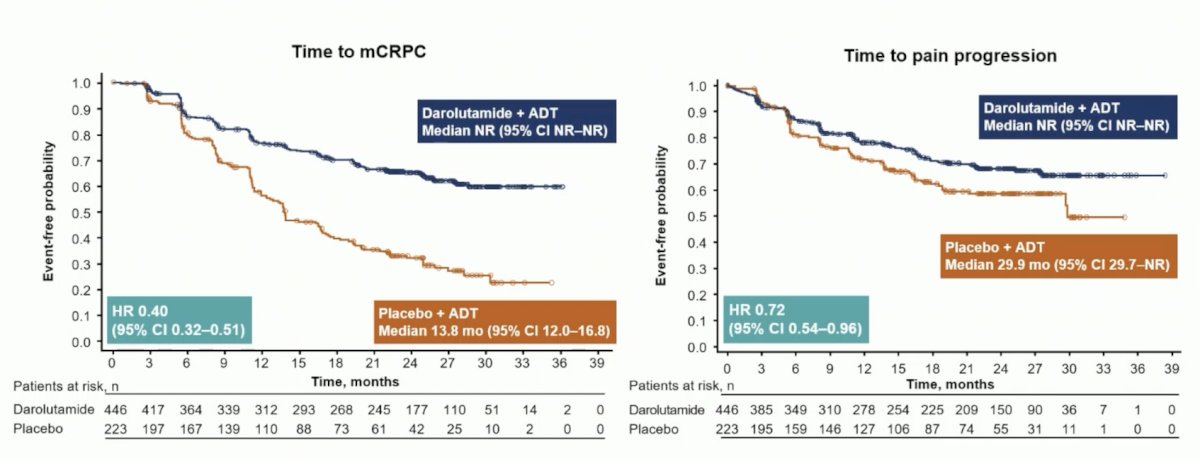
Additionally, there was a benefit favoring darolutamide + ADT for time to subsequent therapy (HR 0.40, 95% CI 0.29–0.56) and time to PSA progression (HR 0.31, 95% CI 0.23–0.41):
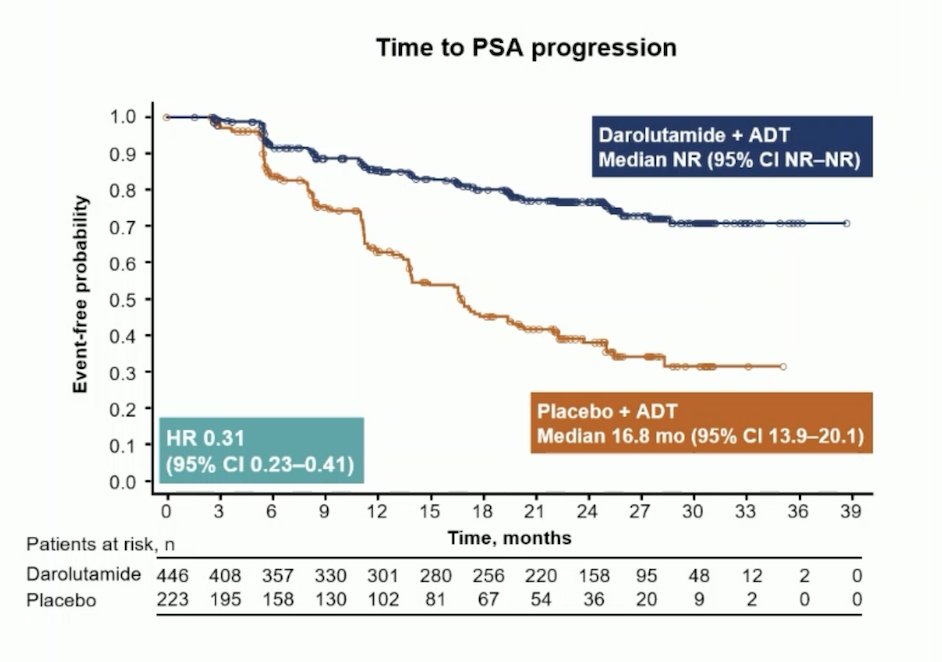
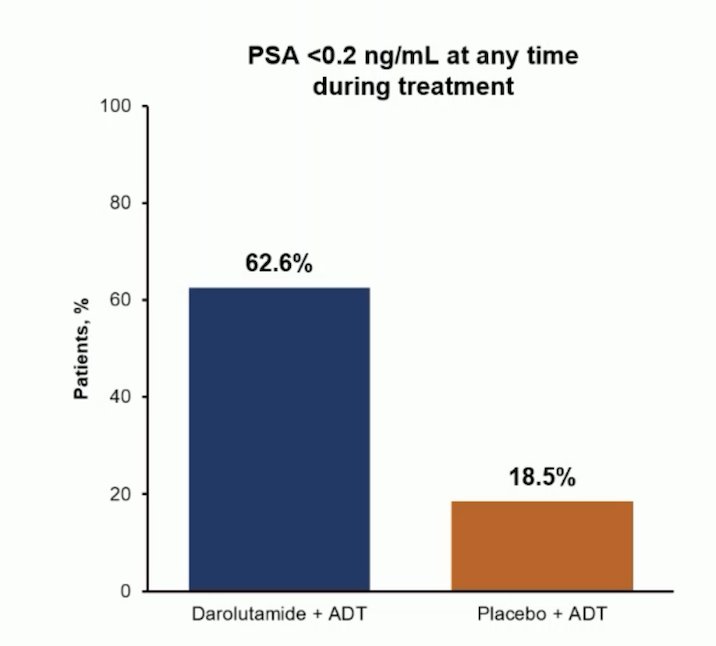
Further, PSA < 0.2 ng/mL at any time during treatment occurred in 62.6% of darolutamide + ADT patients compared to 18.5% for placebo + ADT patients:
A full summary of the secondary outcomes is highlighted in the following table:
Incidences of treatment-emergent adverse events were low and similar between groups, and treatment discontinuations due to treatment-emergent adverse events were lower in patients receiving darolutamide versus placebo (6.1% vs 9.0%):
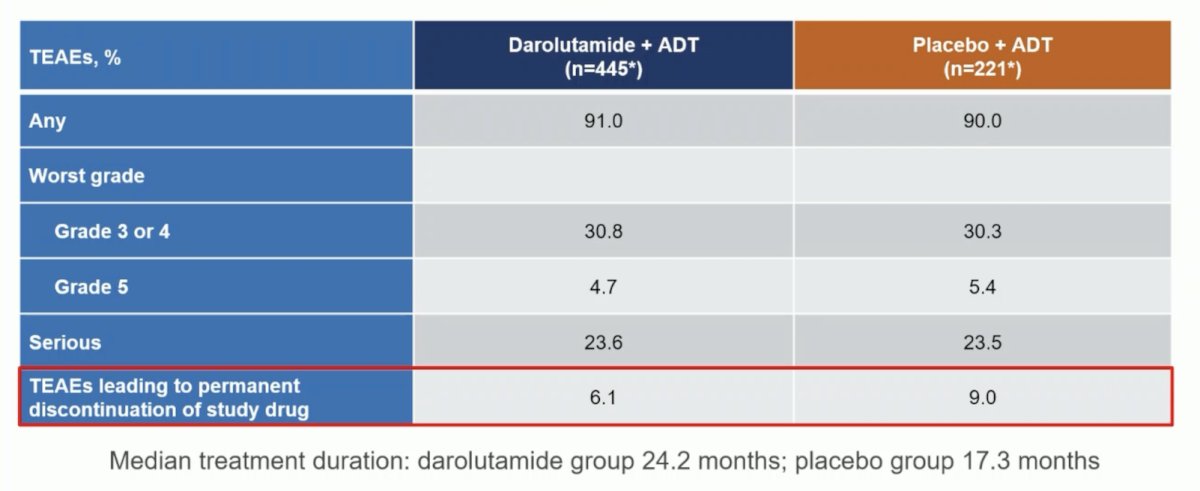
The following table shows that treatment emergent adverse events associated with androgen receptor pathway inhibitors were generally similar between treatment groups; fatigue was less common in the darolutamide + ADT group versus placebo + ADT:

Dr. Saad concluded his presentation by discussing the efficacy and safety of darolutamide plus ADT in patients with mHSPC from the phase 3 ARANOTE trial with the following take-home points:
- Darolutamide + ADT significantly improved radiographic progression free survival in patients with mHSPC
- Darolutamide showed a benefit across all secondary endpoints
- Darolutamide had a favorable safety profile
- Based on these results for ARANOTE, darolutamide + ADT without docetaxel should become an additional standard of care for mHSPC
Presented by: Fred Saad, MD, FRCSC, University of Montreal, Montreal, Quebec, Canada
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:
Related Content:ARANOTE Study: Transforming Metastatic Hormone Sensitive Prostate Cancer Treatment - Fred Saad


