NDO is a common consequence of suprasacral spinal cord injury or spina bifida as an underlying disease. However, other neurological diseases such as multiple sclerosis, ischaemic disease, cerebral palsy, or Parkinson's disease have also been linked to NDO.
It is of the utmost importance to reduce the detrusor leak point pressure to below 40 cm H₂O in order to prevent damage to the upper urinary tract.1
In cases of incomplete bladder emptying due to detrusor underactivity or detrusor sphincter dyssynergia the use of clean intermittent catheterization (CIC) has proven effective. In accordance with the European guidelines on neuro-urology, oral antimuscarinics are recommended as a primary course of treatment for NDO. Second-line treatment of NDO comprises intravesical oxybutynin, followed by botulinum toxin injections and surgery.2
Oral antimuscarinics are frequently associated with significant adverse effects and/or are not sufficiently efficacious. It is therefore evident that alternative safe and effective treatment options for NDO patients are required. The intravesical oxybutynin formulation has been successfully employed in the treatment of adults and children with spinal cord injury and spina bifida for several years. VESOXX® has been the first registered intravesical oxybutynin product in Germany since 2019. Currently, VESOXX® is indicated for the suppression of NDO in children from 6 years of age and adults, who are managing bladder emptying by CIC and if they cannot be adequately managed by oral anticholinergic treatment due to lack of efficacy and/or intolerable side effects, irrespective of the underlying neurological disease. To date, there is limited data on the efficacy and safety of the 2019 approved intravesical oxybutynin therapy (VESOXX®) in NDO patients. The majority of these publications present retrospective case series. In this study, we have collated cases treated with VESOXX® and have designated this approach as intravesical oxybutynin (IO). The objective was to generate data on the safety and efficacy of IO in patients with different underlying diseases, in combination with other treatments, and in relation to the sequence of treatments.
In total, 38 case reports from routine clinical practice were collated by 14 physicians from Germany, the Netherlands, and Switzerland. The case collection encompasses 18 patients below the age of 18 and 20 adult patients. The parameters reported include detrusor pressure (Pdetmax), maximum bladder capacity (MBC), incontinence periods, urinary tract infections, bladder morphology, and safety, as well as the severity of adverse events.
The results are mainly of descriptive value. Paired Student’s t-tests were performed to eliminate potential differences between the observers.
The data were aggregated into distinct groups according to etiology, age (<18 years and ≥18 years), and preceding therapies (Tables 1 and 2).
Table 1: Patient groups divided according to etiology of underlying disease. SCI, spinal cord injury; SB, Spina Bifida; IO, intravesical oxybutynin; BTX, botulinum toxin A.
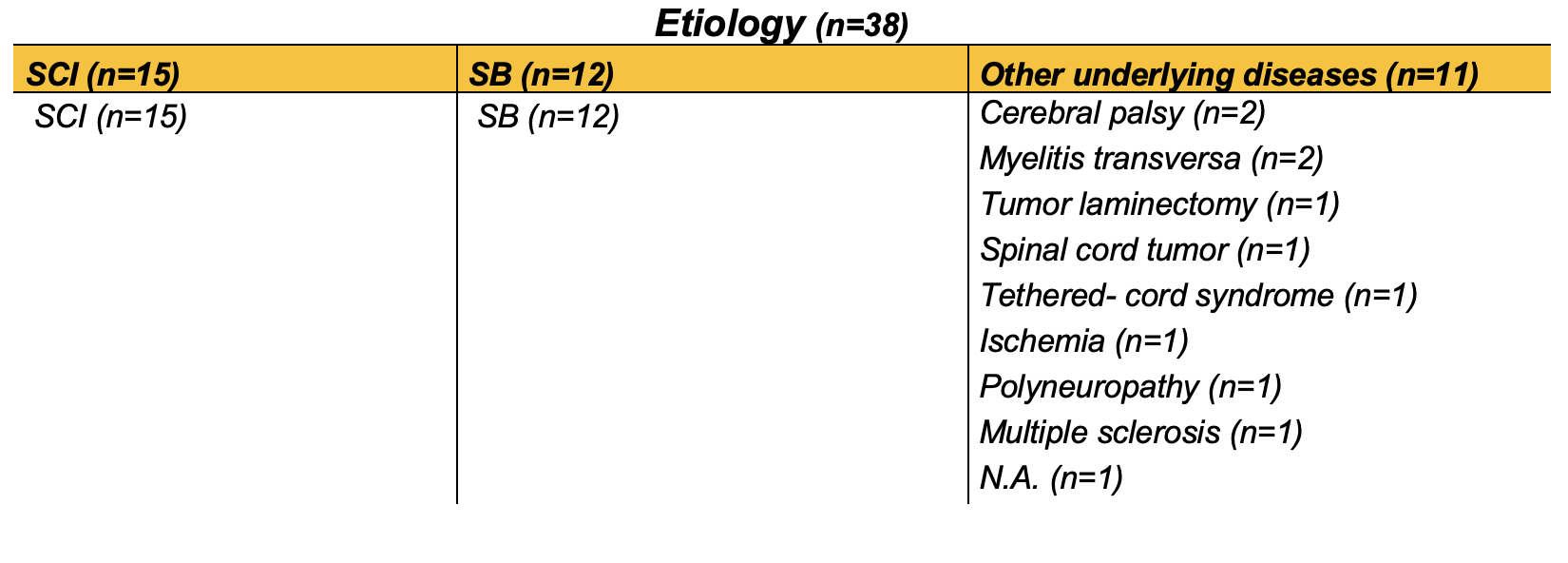
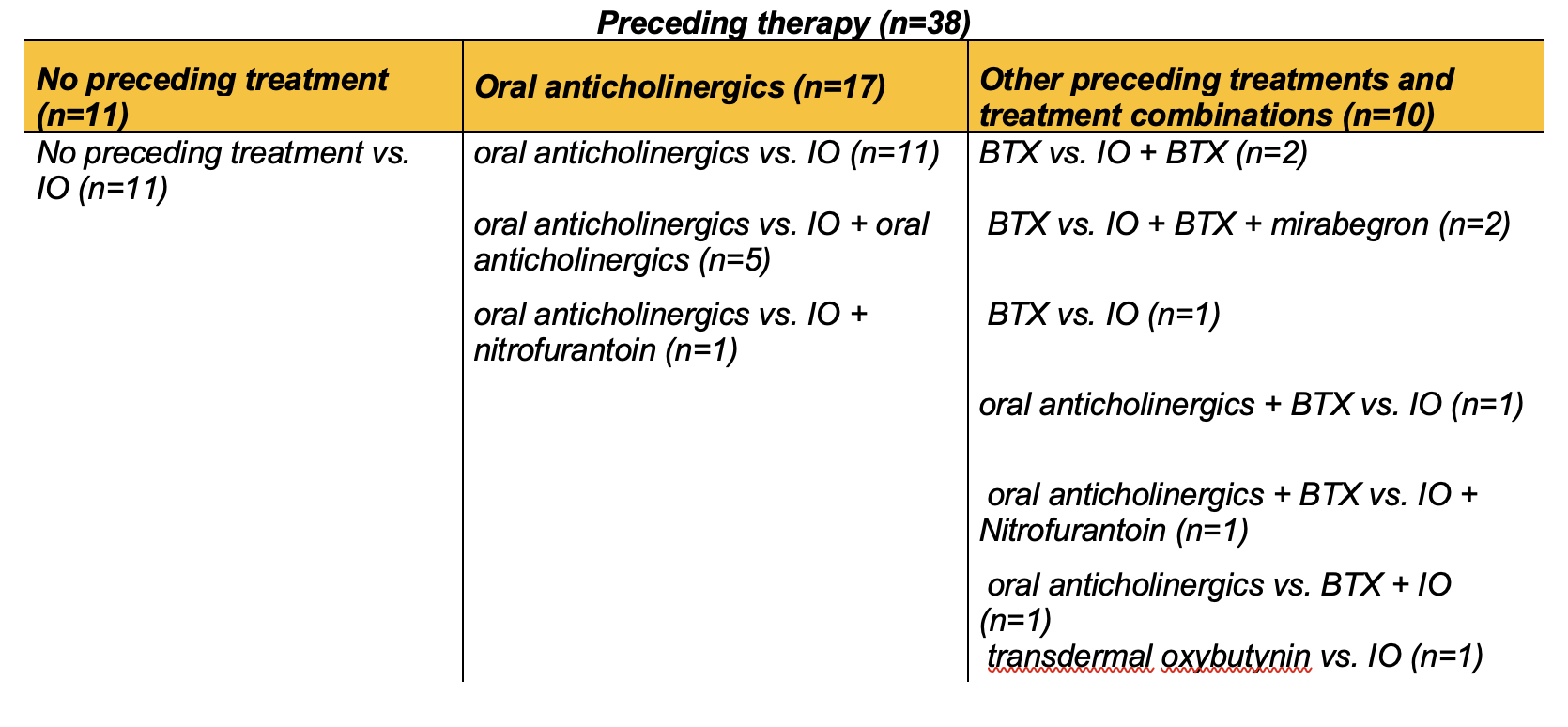
Table 2: Patient groups sorted by preceding treatment before intravesical oxybutynin partly with combination therapies. SCI, spinal cord injury; SB, Spina Bifida; IO, intravesical oxybutynin; BTX, botulinum toxin A.
Pdetmax exhibited a notable decline, with a reduction of 59% observed from a baseline of 51.94 cm H2O (± 26.12 SD) to 21.07 cm H2O (± 17.32 SD) following the administration of IO (Fig. 1). This change was statistically significant (***P <0.001, n=34). Significant changes in Pdetmax were observed in patients below (59.06 cm H2O to 23.41 cm H2O 60% change **P<0.01) and above the age of 18 (44.82 cm H2O to 21.69 52% change *** P <0.001). Furthermore, a sub-analysis of the data for SCI and other underlying diseases revealed a significant reduction in Pdetmax. In the case of SB, the reduction exhibited a tendency, though it did not reach a statistically significant level (Fig. 1).

Figure 1: Maximum detrusor pressure (Pdetmax) of NDO patients before and with IO treatment. All patient groups (A), age (B), etiology (C), and prior treatment to IO treatment (D). Paired Student's t-tests; n.s. P>0,05; *P<0,05; **P<0,01; ***P <0,001. Pdet max., maximum bladder pressure; IO, intravesical oxybutynin; SCI, spinal cord injury; SB, spina bifida
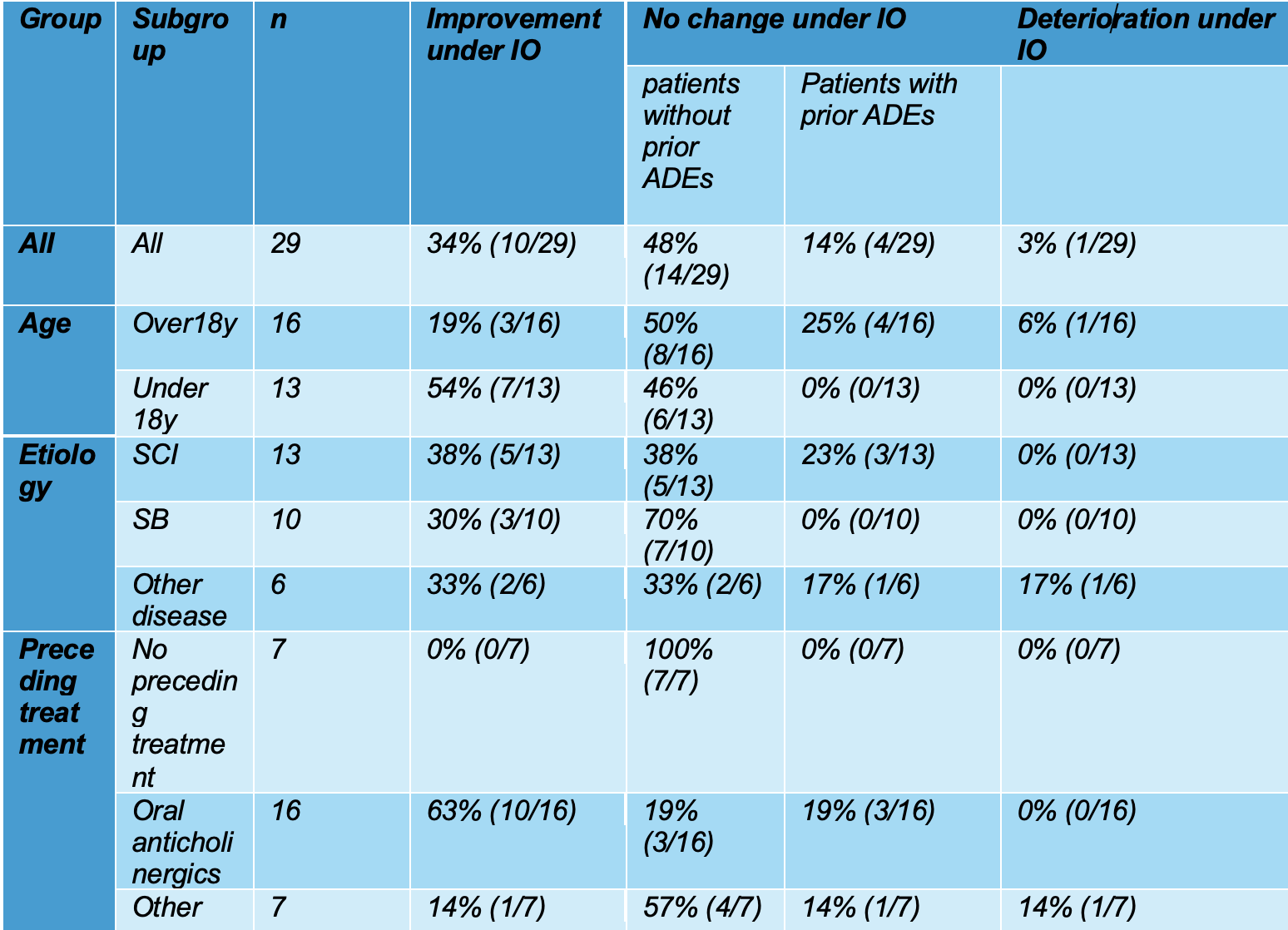
MBC exhibited a statistically significant increase of 34% from 260.45 ml ± 200.26 SD to 348.45 ml ± 175.90 SD. Once more, the results of the sub-analyses indicated significant discrepancies between the subgroups comprising patients below and above the age of 18 (see Fig. 2).
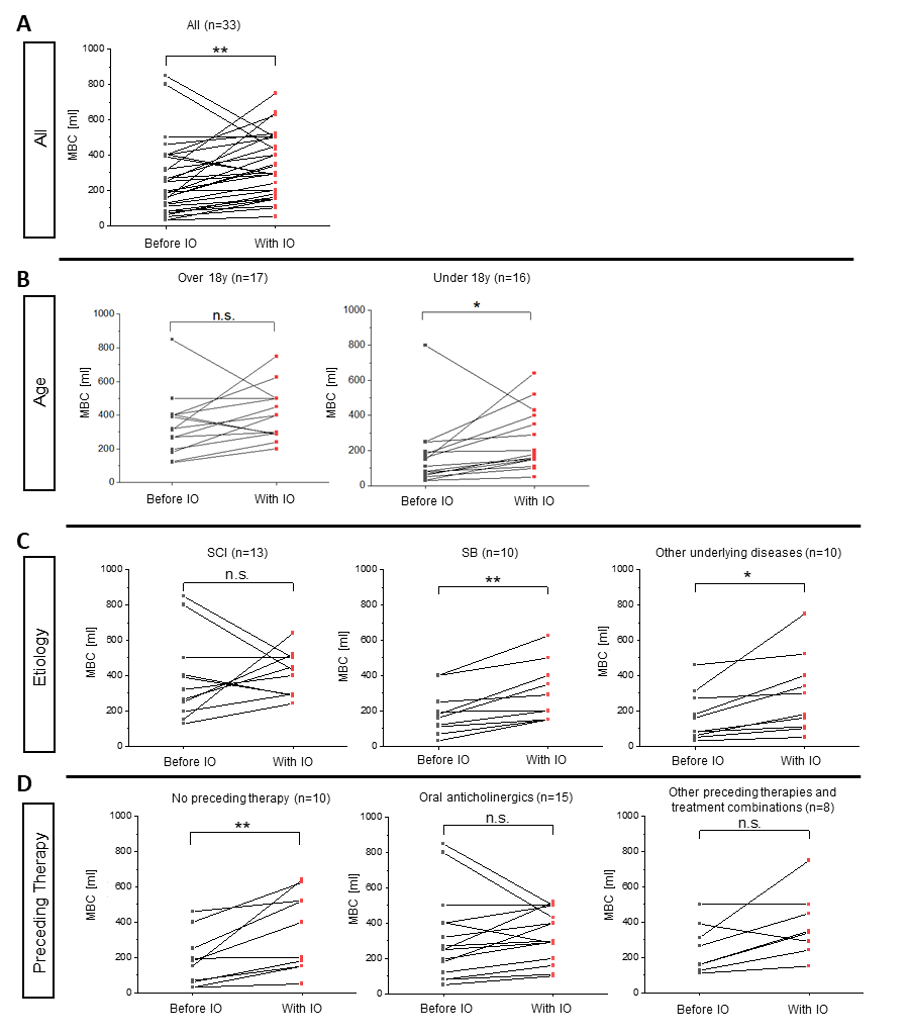
Figure 2: Maximum bladder capacity (MBC) of NDO patients before and with IO treatment. All patient groups (A), age (B), etiology (C), and prior treatment to IO treatment (D). Paired Student's t-tests; n.s. P>0,05; *P<0,05; **P<0,01; ***P <0,001. Pdet max., maximum bladder pressure; IO, intravesical oxybutynin; SCI, spinal cord injury; SB, spina bifida
Improvements in bladder morphology were observed in 44% of patients. In 50% of cases, there was no change, while in only two patients, a deterioration was observed.
The frequency of urinary incontinence decreased in the majority of patients following the commencement of IO treatment, with no increase in incontinence episodes observed in any patient.
Prior to initiating intravesical oxybutynin IO therapy, 15.8% of patients were continent, whereas following IO therapy, this figure increased to 55.3%.
The frequency of urinary tract infections (UTIs) improved in 36% of patients and remained unchanged in 61%. Only one patient experienced an increased frequency of UTIs.
It was observed that adverse drug events (ADEs) were reported in 82% of patients who were treated with oral anticholinergics. In 63% of patients with ADEs, a change in treatment to IO resulted in an improvement in the ADEs. No increase in ADEs was observed following a therapeutic shift towards IO. In other subgroup analyses, improvements regarding ADEs ranged from 33% to 100% (Table 3). In total, only one patient reported an exacerbation of ADEs.
Table 3: Changes in ADE severity before and after IO. Ach, anticholinergics; SCI, Spinal cord injury; SB, Spina bifida. Group Subgroup n Improvement under IO No change under IO Deterioration under IO patients without prior ADEs Patients with prior ADEs
There is no standard dosage for intravesical oxybutynin, the summary of product characteristics (SmPC) of VESOXX® states maximum dosages of 30 mg for children and elderly and 40 mg for adult patients. Guidelines suggest for children dose adaptations between 0.1-0.9 mg/kg body weight. Consequently, data were also gathered from routine clinical practice regarding dosages and the typical frequency of administration. The mean daily dose for adults was 23.6 mg, with a frequency of 2.3 applications per day. For children and adolescents under 18 years of age, the mean daily dose was 17.1 mg, divided into 2.7 applications per day. In relation to body weight, daily dosages ranged from 0.54 to 0.9 mg/kg body weight.
In conclusion, IO was demonstrated to be both efficacious and safe in this series of cases. The results demonstrated that IO was more effective than previous oral anticholinergic treatments in a number of categories, including Pdetmax, MBC, bladder morphology, incontinence frequency, urinary tract infections, and ADEs.
In a prospective, randomised study with 35 adults, Schröder et al. (2016) reported on the efficacy, safety, and tolerability of IO compared to oral oxybutynin. An increase in MBC and a reduction in the incidence of ADEs were observed.3 Shen et al. conducted a meta-analysis and review to evaluate the efficacy and safety of intravesical oxybutynin. A total of 19 studies were included in the analysis, comprising 392 cases of children and adults with NDO who had previously responded poorly to oral anticholinergics or experienced severe adverse effects.4 The authors demonstrated that IO is an effective method for reducing Pdetmax, improving MBC and continence while causing fewer side effects. The authors concluded that IO treatment represents a viable option for both adults and children, given its favourable efficacy and fewer adverse effects compared to oral oxybutynin treatment. Furthermore, the long-term use of IO in children up to the age of 15 has been demonstrated to be an effective treatment option with a favourable side effect profile.5 These findings align with our observations from the treatment routine for Pdetmax and MBC. In all subgroups, a Pdetmax of less than 40 cm H₂O was observed, which is considered to be a safe detrusor pressure. No statistically significant change was observed in the SB patients. It should be noted, however, that this group of patients exhibited a relatively low Pdetmax of 41.6 cm H2O on average prior to IO therapy. This is consistent with the findings of the study by Richard et al., which compared urological parameters between SCI, multiple sclerosis (MS), and SB patients diagnosed with NDO. Pdetmax was significantly lower in the SB group (54.1 cm H2O in SCI, 41.2 cm H2O in MS, and 29.3 cm H2O in SB).6
Furthermore, MBC increased significantly. Bladder morphology, frequency of incontinence episodes, and urinary tract infections for the most part also improved or remained the same in the SB group after switching to IO. The increase in bladder capacity in the <18-year-olds was 103.56 ml. In light of the observed average annual increase in MBC of 25 ml in children, and the median interval of 10 months between the urodynamic measurements, the adjusted increase in bladder capacity was 82.73 ml. It is important to note that the assessment of bladder morphology is based on a subjective evaluation.7-10
The daily dosage for children ranged from 0.54 to 0.9 mg per kilogram of body weight. This is in alignment with the upper end of the recommended guidelines. In general, there are two justifications for the administration of a dosage:
- Avoidance of damage to the upper urinary tract
- Minimization of adverse drug reactions
Limitations of this retrospective case series are the observational nature, the small number of cases, and the heterogeneity of the patient cohort. The connecting factor in this case series was the neurogenic detrusor overactivity, which was the decisive factor for treatment with IO in this collective. Furthermore, the statistical tests are only indicative due to the observational nature and the small number of cases.
The present case summary shows the efficacy and safety of IO in a variety of different therapeutic situations in daily practice in SCI, SB, and patients with other underlying diseases, either alone or in combination with other treatment options as well as in atypical therapeutic cascades, which have not yet been recorded in the current literature is noteworthy that this report details the use of IO in the treatment of cerebral palsy, myelitis transverse, spinal cord tumours, ischaemia, multiple sclerosis, and polyneuropathy, some of which have not yet been described in the literature.
The first-line use of IO in patients under 18 years of age may be explained by administration problems, as it occurs in infants and young children with oral tablets. As the cases were collected in daily practice, i.e. without intervention, the data represent realistic scenarios with preceding and concomitant therapies, but the use of IO in this way should still be classified as off-label.
Take home message:
This case series supports the use of IO according to the current European and German guidelines for the treatment of NDO patients as second-line therapy after oral anticholinergics and before treatment with botulinum toxin injections. IO significantly reduced the average Pdet max, increased the average MBC, and reduced the frequency of incontinence episodes and side effects in patients. IO therapy can be used in patients who are refractory to oral therapy or in combination with various other therapeutics and as a complementary treatment to botulinum toxin injections. However, the use of combination therapies and non-typical treatment sequences such as botulinum toxin injections followed by IO should be investigated in larger populations. Experience is also available on various etiologies of NDO and the use of IO.
The approved IO preparation VESOXX® may be a useful alternative for patients who do not respond to other therapies or suffer from side effects.
Written by: O. Schindler,1 H. Ho,2 Q. Leidl,2 A. Angermund,3 R. Elishar,4 M. Frech-Dörfler,5 A. Hirsch,6 Y.-B. Kalke,7 R. Kirschner-Herrmanns,8 J. Tornic,9 F. Queissert,10 S. Rahnama'i,11 C. Rehme,12 A. Reitz,13 F. Schmitz,14 D. Schultz-Lampel,15 M. Gedamke2
- Spinal Cord Injury Center of the Orthopedic University Hospital Ulm, Ulm, Germany
- FARCO-PHARMA GmbH, Cologne, Germany
- Centre for Neuro-Urology, Schön Klinik Vogtareuth, Vogtareuth, Germany
- Klinikum Bayreuth GmbH, Bayreuth, Germany
- Universitäts-Kinderspital beider, Basel, Switzerland
- Cnopfsche Kinderklinik, Nürnberg, Germany
- Johanniter Rehabilitationszentrum Godeshöhe, Bonn-Godesberg, Germany
- Department of Urology, Kantonsspital Wintherthur, Winterthur, Switzerland
- Department of Urology and Children’s Urology, University Hospital Münster, Germany
- Department of Urology and Children’s Urology, Aachen, Germany
- Department of Urology, University Hospital Essen, Essen, Germany
- KontinenzZentrum AG, Klinik Hirslanden, 8032 Zürich, Switzerland
- Department of Urology and Children’s Urology, University Hospital Ulm, Ulm, Germany
- Neuro-Urologie/Urologie und Kinderurologie, Universitätsklinikum Bonn
- Continence CenterSouthwest, Schwarzwald-Baar Klinikum, Villingen-Schwenningen, Germany
References:
- Leslie, S.W., P. Tadi, and M. Tayyeb, Neurogenic Bladder and Neurogenic Lower Urinary Tract Dysfunction, in StatPearls. 2023: Treasure Island (FL).
- Blok, B., et al., EAU Guidelines on Neuro-Urology. EAU Guidelines Office, 2023(Edn. presented at the EAU Annual Congress Milan 202).
- Schroder, A., et al., Efficacy, safety, and tolerability of intravesically administered 0.1% oxybutynin hydrochloride solution in adult patients with neurogenic bladder: A randomized, prospective, controlled multi-center trial. Neurourol Urodyn, 2016. 35(5): p. 582-8.
- Shen, S.H., et al., Intravesical oxybutynin therapy for patients with neurogenic detrusor overactivity: a systematic review and meta-analysis. Int Urol Nephrol, 2022. 54(4): p. 737-747.
- Humblet, M., et al., Long-term outcome of intravesical oxybutynin in children with detrusor-sphincter dyssynergia: with special reference to age-dependent parameters. Neurourol Urodyn, 2015. 34(4): p. 336-42.
- Richard, C., et al., Urinary biomarkers profiles in patients with neurogenic detrusor overactivity according to their neurological condition. World J Urol, 2020. 38(9): p. 2261-2268.
- Abrams, P., et al., The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology, 2003. 61(1): p. 37-49.
- Hjalmas, K., et al., Nocturnal enuresis: an international evidence-based management strategy. J Urol, 2004. 171(6 Pt 2): p. 2545-61.
- Koff, S.A., Estimating bladder capacity in children. Urology, 1983. 21(3): p. 248.
- Seibold, J., et al., Presenting a New Non-Invasive Diagnostic Tool in Estimating Bladder Capacity in Healthy Children: The Abc (Age-Related Bladder Capacity)-Formula. Journal of Pediatric Urology, 2010. 6: p. S100-S101.
- Gedamke, M., Y. Steffen, and Q. Leidl, Comparison of adverse events (AE) of different treatments for neurogenic detrusor overactivity (NDO). Deutsche Kontinenz Gesellschaft e.V.. 34. Kongress der Deutschen Kontinenz Gesellschaft. Leipzig, 03.-04.11.2023. Düsseldorf: German Medical Science GMS Publishing House, 2023.


