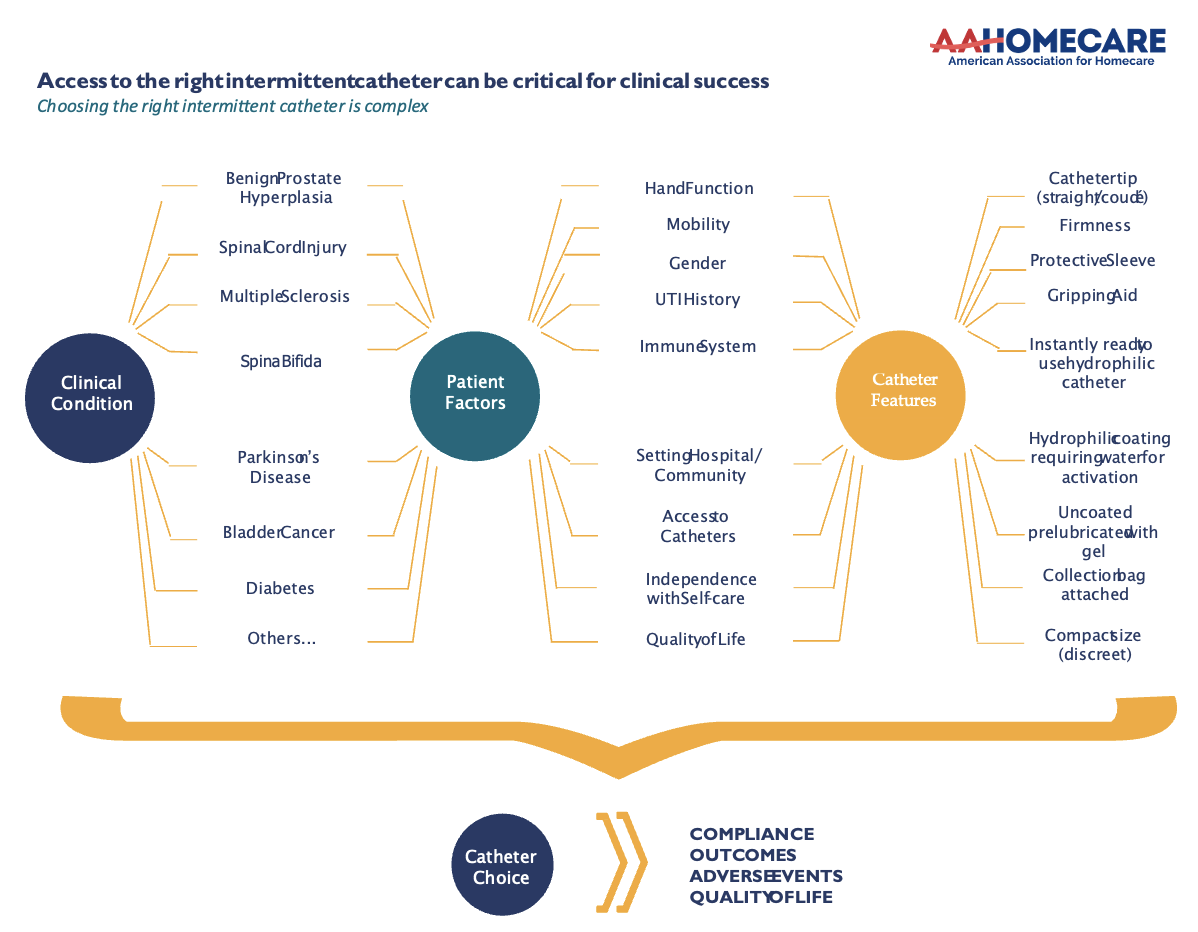
But catheter technology has outpaced current HCPCS (Healthcare Common Procedure Coding System, medical codes that help health insurance providers process claims for medical supplies, like ICs, as they are used by Medicare (CMS), Medicaid, and other insurers to ensure claims are processed consistently). The growth in catheter material, types, etc has evolved over decades. There are over 1,300 products available but there are only three HCPCS codes available for reimbursement or coverage.
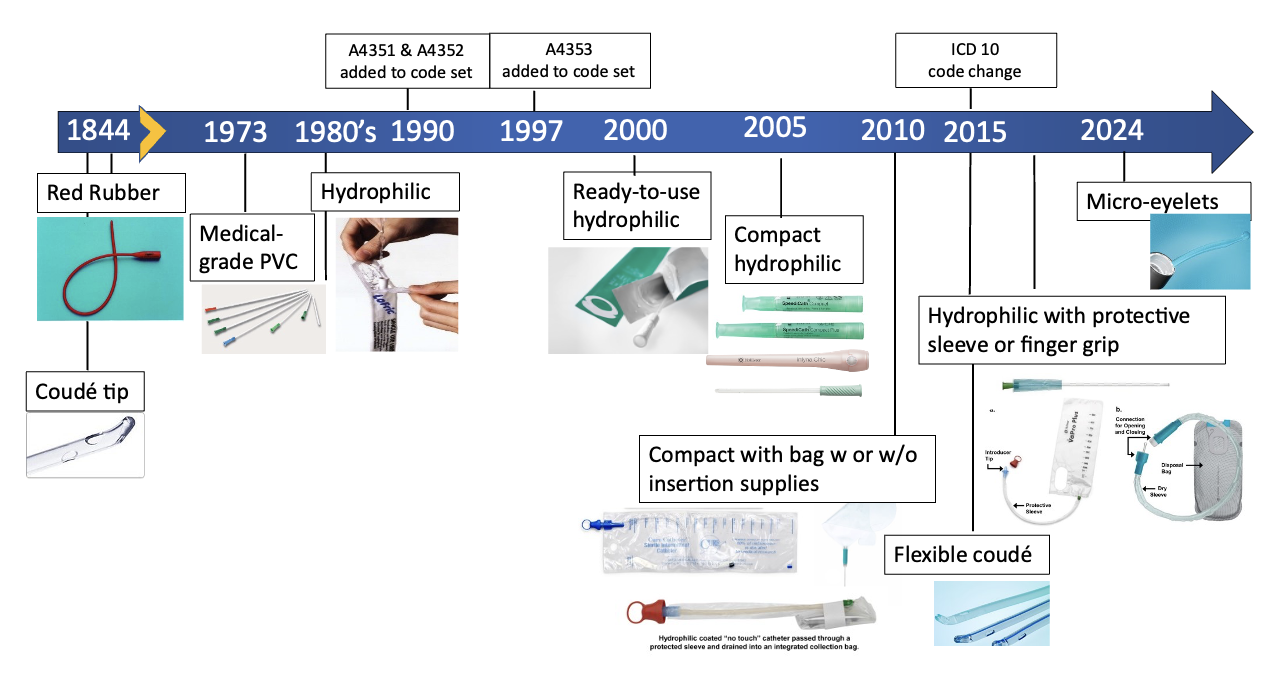
This is going to change as CMS releases final HCPCS coding decisions for payment of ICs, which will become effective on January 1, 2026.
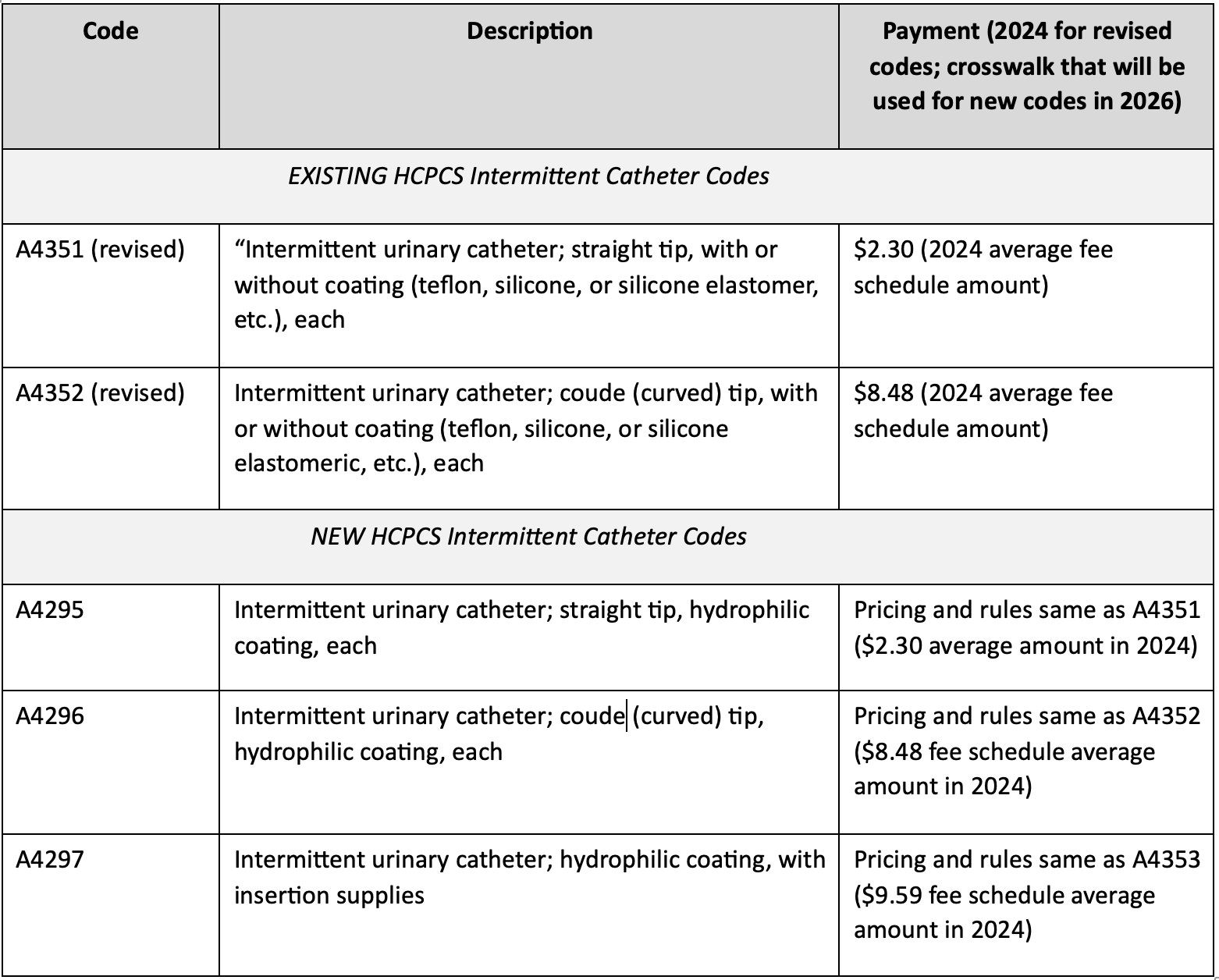
They revised 2 existing codes to remove the word “hydrophilic” and have created 3 new codes specific to catheters with “hydrophilic coating”. They are 1) hydrophilic straight tip catheter, 2) hydrophilic Coudé tip catheter, and 3) hydrophilic catheter with insertion supplies.
CMS provided a definition for hydrophilic: “The hydrophilic coating on a catheter is intrinsic to the catheter product (i.e., you cannot wipe the coating off).” CMS noted that a hydrophilic catheter is a type of (not the same as) pre-lubricated catheter having a polymer coating that binds water to the catheter to make it slippery. However, CMS noted there was no clinical evidence to support the claim that urinary tract infection reduction benefits provided by hydrophilic catheters are either matched or exceeded by pre-lubricated catheters.
Payment for the new codes maps to payment for existing codes. The table below shows the final codes, descriptions, and payment rates.
Written by: Diane K. Newman, DNP, ANP-BC, FAAN is an Adjunct Professor of Urology in Surgery, Perelman School of Medicine, University of Pennsylvania and Former Co-Director of the Penn Center for Continence and Pelvic Health.
References:
- Averch T.D., Goldman HB, Griebling T., Newman D.K., et al. AUA Indwelling urinary catheter management of the acute patient, Quality improvement issue brief. 2024. White Paper on Catheter-Associated Urinary Tract Infections: Definitions and Significance in the Urologic Patient. 2024, https://www.auanet.org/guidelines-and-quality/quality-and-measurement/quality-improvement/clinical-consensus-statement-and-quality-improvement-issue-brief-(ccs-and-qiib)/indwelling-urinary-catheter-management-of-the-acute-patient
- Averch T.D., Stoffel J., Goldman H.B., Griebling T., Lerner L., Newman D.K., Peterson A.C. AUA White Paper on Catheter-Associated Urinary Tract Infections: Definitions and Significance in the Urologic Patient. 2014.
- Cardenas, D.D., et al. Intermittent catheterization with a hydrophilic-coated catheter delays urinary tract infections in acute spinal cord injury: a prospective, randomized, multicenter trial. PM R. 2011; 3:408-417
- Chartier-Kastler, E. & Denys, P. Intermittent catheterization with hydrophilic catheters as a treatment of chronic neurogenic urinary retention. Neurourol Urodyn 30, 21-31 (2011).
- Goetz, L.L., Droste, L., Klausner, A.P. & Newman, D.K. Catheters Used for Intermittent Catheterization. in Clinical Application of Urologic Catheters, Devices and Products. 2018; 47-77.
- Kohler TS, Yadven M, Manvar A, Liu N, Monga M. The Length of the Male Urethra. International Braz J Urol. 2008; 34(4):451-456.
- Newman, D.K., et al. Intermittent catheterization with single- or multiple-reuse catheters: clinical study on safety and impact on quality of life. Int Urol Nephrol. 2020; 52: 1443-1451.
- Newman D.K., Wilson, M.M. Review of intermittent catheterization and current best practices. Society of Urologic Nurses and Associates, Urol Nurs, 2011; 48:12-29.
- Newman D.K. Methods and Types of Urinary Catheters Used for Indwelling or Intermittent Catheterization. Urol Nurs. 2021; 41(2):111-117. https://www.suna.org/sites/default/files/download/resources/SUNA_cathetersTool.pdf
- Prieto JA, Murphy CL, Stewart F, Fader M. Intermittent catheter techniques, strategies and designs for managing long-term bladder conditions. Cochrane Database Syst Rev. Oct 26 2021;10:CD006008. doi:10.1002/14651858.CD006008.pub5
- Vahr S, Chagani S, Daniels A, Kelly T, et al. Catheterisation: Urethral Intermittent in Adults including urethral intermittent dilatation. Evidence based guidelines for best practice in urological health care European Association of Urology Nurses; 2024, https://nurses.uroweb.org/wp-content/uploads/EAUN-Guideline-Urethral-intermittent-catheterisation-in-adults-2024.pdf


