(UroToday.com) The 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain was host to the presentation of poster 1611. Dr. Kim Chi discussed the hematologic impact of [177Lu]Lu-PSMA-617 versus androgen receptor pathway inhibitor (ARPI) change in patients with metastatic castration-resistant prostate cancer (mCRPC) in the PSMAfore trial.
The PSMAfore trial (NCT04689828), was a phase 3 trial of 177Lu-PSMA-617 in taxane-naive patients with mCRPC versus ARPI change in taxane-naive patients with PSMA-positive mCRPC. Patients included in the PSMAfore trial had mCRPC and were candidates for ARPI change after one progression on prior ARPI, and had at least 1 PSMA-positive lesion and no exclusionary PSMA-negative lesions as determined by 68Ga-PSMA-11 PET/CT.1
Patients were randomized 1:1 to receive either open-label 177Lu-PSMA-617 (7.4 GBq every 6 weeks for 6 cycles) or an ARPI change (abiraterone or enzalutamide). Importantly, patients randomized to ARPI could crossover to 177Lu-PSMA-617 following blinded independent centrally reviewed (BICR) confirmation of radiographic progression. The PSMAfore trial showed that 177Lu-PSMA-617 prolonged radiologic progression free survival (rPFS) versus ARPI change with a favourable safety profile. Dr. Chi reported the incidence, risk factors, and management of hematologic treatment emergent adverse events (TEAEs) associated with 177Lu-PSMA-617.
Hematologic treatment-emergent adverse events (TEAEs) were classified according to Common Terminology Criteria for Adverse Events (CTCAE) v5.0 and grouped using MedDRA v26.1 terms. Metastatic bone disease at baseline was evaluated through bone scans. Hematology assessments were conducted at baseline, during treatment, and at follow-up. This safety analysis utilized data from the safety analysis set (227 participants randomized to 177Lu-PSMA-617 and 232 participants randomized to ARPI change), with a data cutoff date of February 27, 2024. The median time from randomization to the data cutoff (full analysis set) was 24.11 months in the 177Lu-PSMA-617 arm and 24.13 months in the ARPI change arm.
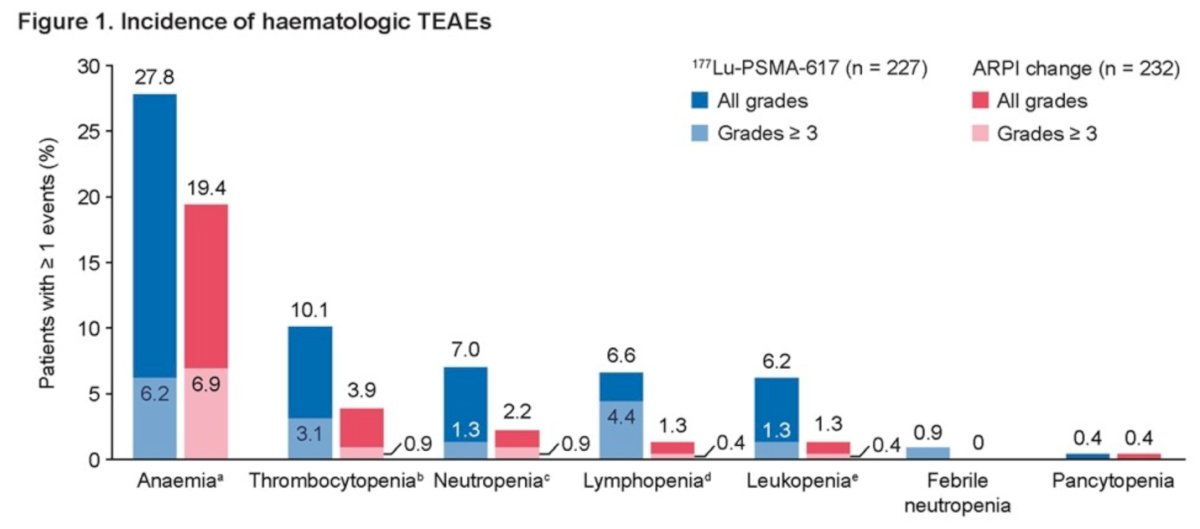
Dr. Chi reported that the incidence of hematologic treatment-emergent adverse events (TEAEs) was higher among patients receiving 177Lu-PSMA-617 compared to those receiving ARPI change (37.9% vs. 23.3%, respectively). Additionally, Grade 3 hematologic TEAEs were more frequently observed in patients treated with 177Lu-PSMA-617 than in those receiving ARPI change (23.3% vs. 8.6%). The most common hematologic TEAEs observed in patients treated with 177Lu-PSMA-617 were:
- Anemia: 27.8%
- Thrombocytopenia & decreased platelet count: 10.1%
- Neutropenia, decreased neutrophil count, and febrile neutropenia: 7.9%
- Lymphopenia & decreased lymphocyte count: 6.6%
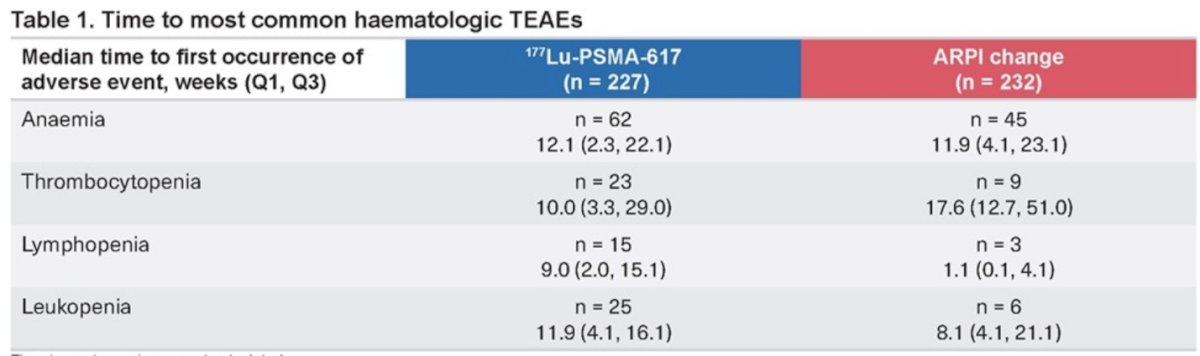
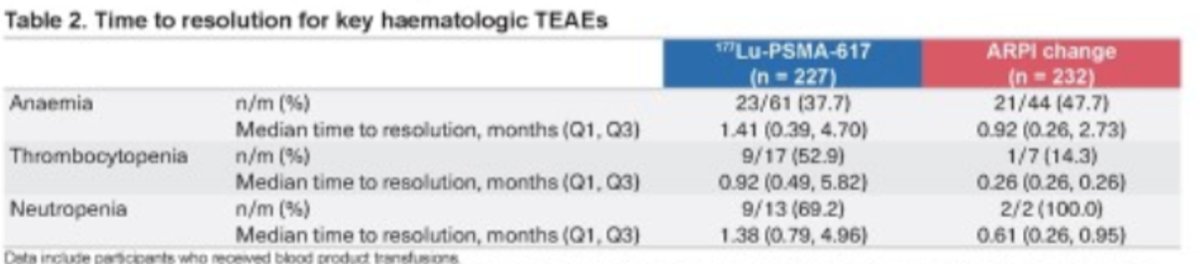
The median time to the most common hematologic treatment-emergent adverse events (TEAEs) is illustrated in the table below. It was similar between the 177Lu-PSMA-617group and the ARPI change group for anemia (12.1 months vs. 11.9 months). However, it was longer in the ARPI change group for thrombocytopenia (17.6 months vs. 10 months).
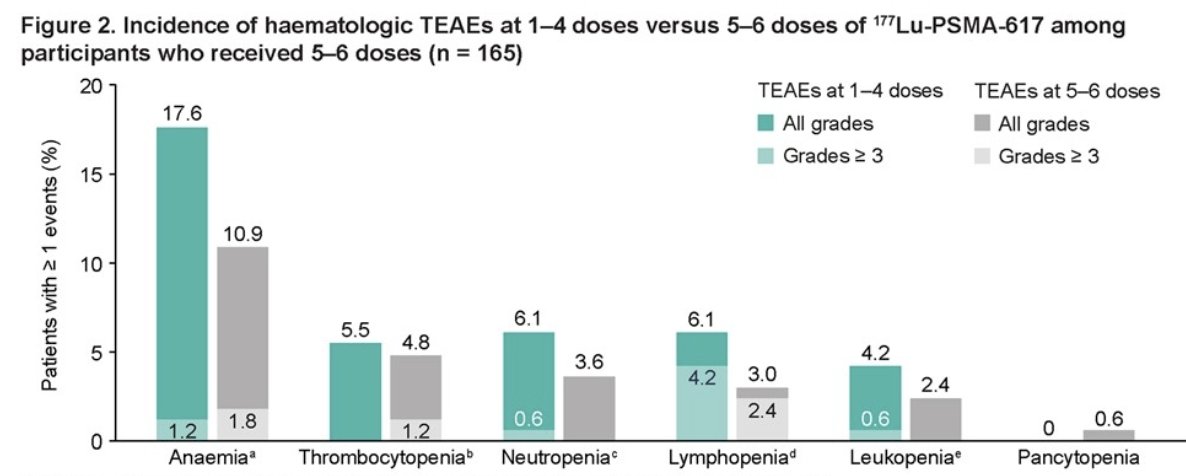
Among participants who received 5-6 doses of 177Lu-PSMA-617, the overall incidence of hematologic treatment-emergent adverse events (TEAEs) was lower for those receiving 5-6 doses (20.0%) compared to those receiving 1-4 doses (29.7%). This trend was observed for all hematologic TEAEs, including anemia, thrombocytopenia, neutropenia, and lymphopenia, as illustrated in the graphic below:
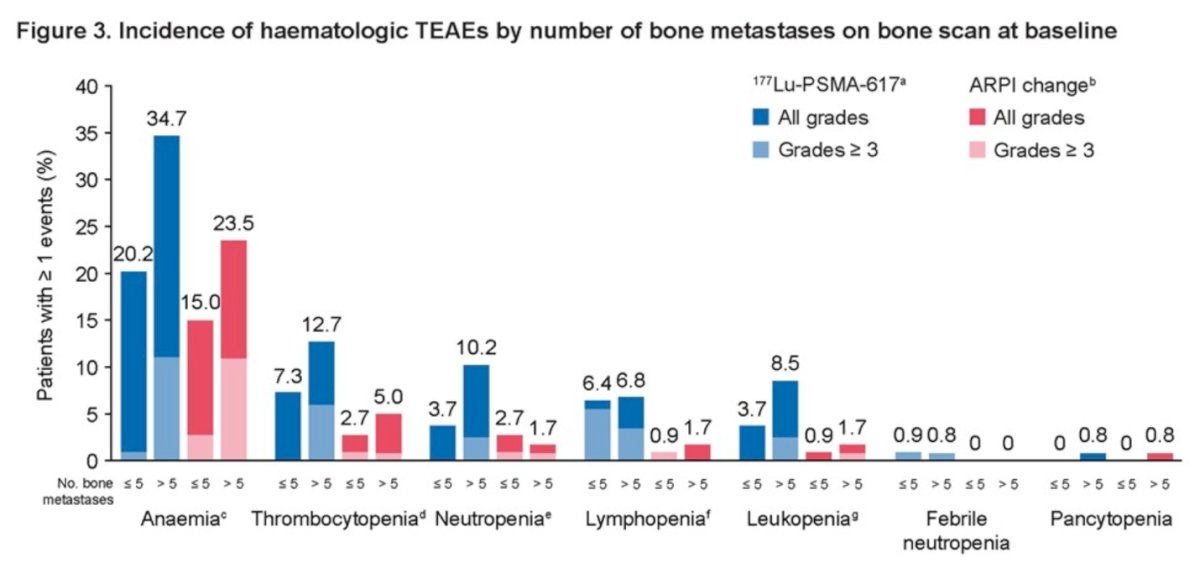
For 177Lu-PSMA-617 versus ARPI change, hematologic TEAE incidences were 30.3% versus 19.5% in patients with ≤5 metastatic bone lesions and 44.9% versus 26.9% in patients with >5 bone lesions. Interestingly, the incidence of hematologic TEAEs did not increase for patients receiving 5/6 versus 1–4 doses of 177Lu-PSMA-617.
The incidence of any grade and grade ≥ 3 hematologic TEAEs was higher in participants with > 5 bone metastases (44.9% vs. 30.3%) in the 177Lu-PSMA-617 group compared to those with ≤ 5 bone metastases, and (26.9% vs. 19.5%) in the ARPI change group.
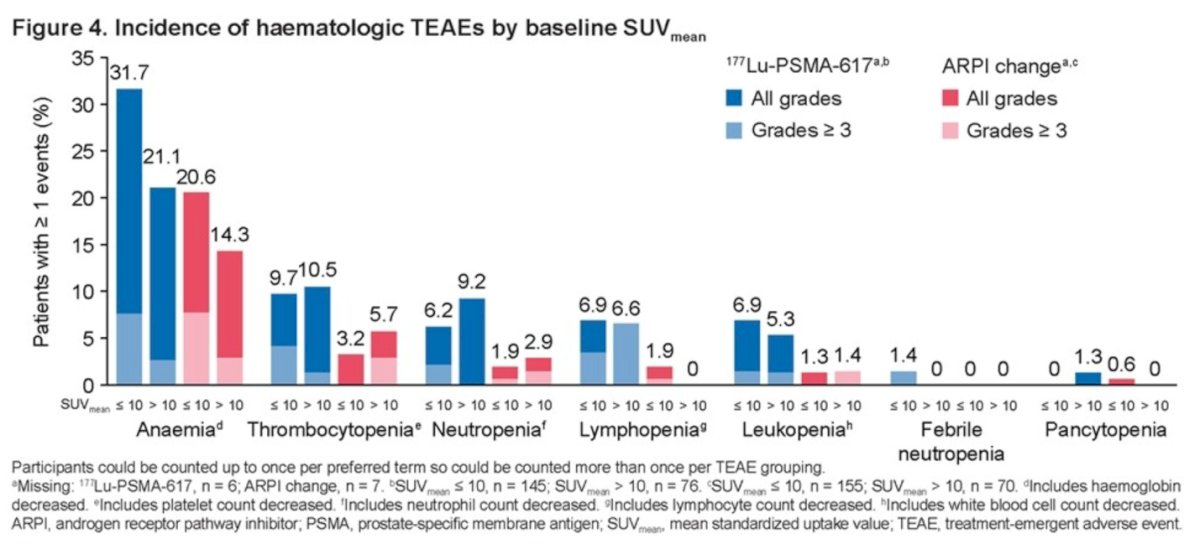
Hematologic TEAEs were also more common in participants with SUVmean ≤ 10: 40% in the 177Lu-PSMA-617 group versus 24.5% in the ARPI change group, compared to 34.2% in the 177Lu-PSMA-617 group versus 18.6% in the ARPI change group for patients with SUVmean > 10.
The median time to resolution of hematologic TAEs was always longer in the 177Lu-PSMA-617 group vs. the ARPI change group as illustrated in the table below:
To close his presentation, Dr. Chi made the following observations:
- The incidence of hematologic treatment-emergent adverse events (TEAEs) was higher in patients receiving 177Lu-PSMA-617 compared to those in the ARPI change arm (37.9% vs. 23.3%).
- Despite this higher incidence, the rates of severe complications or the need for management interventions, such as blood transfusions, erythropoietin use, or granulocyte colony-stimulating factors, were low and comparable between the two treatment arms.
- Among participants who received 5-6 doses of 177Lu-PSMA-617, TEAEs were more frequent at 1-4 doses than at 5-6 doses suggesting the risk of hematologic TEAs does not increase with incremental dosing.
- In both study treatment arms, participants with a higher number of bone metastases (>5) and lower SUVmean (<10) at baseline had a higher rate of hematologic TEAEs.
Presented by: Kim N. Chi, MD, British Columbia Cancer Agency, Vancouver Cancer Centre, Vancouver, Canada
Written by: Julian Chavarriaga, MD – Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:- LBA13 Phase III trial of [177Lu]Lu-PSMA-617 in taxane-naive patients with metastatic castration-resistant prostate cancer (PSMAfore) Sartor, O. et al. Annals of Oncology, Volume 34, S1324 - S1325

