Introduction
At the ESMO 2024 annual meeting in Barcelona, Spain, the results of numerous practice-changing trials were presented, including those from three highly-anticipated clinical trials in the advanced prostate cancer space:
- ARANOTE in metastatic hormone sensitive prostate cancer (mHSPC)
- PEACE-3 and SPLASH in metastatic castration resistant prostate cancer (mCRPC).
ARANOTE
Why is this trial important?
Darolutamide has been previously approved for mHSPC in combination with docetaxel and ADT based on results of the phase 3 ARASENS trial.1 In ARASENS, darolutamide + ADT + docetaxel significantly improved overall survival (OS) versus ADT + docetaxel (HR 0.68, 95% CI 0.57–0.80) in patients with mHSPC, and the incidences of treatment-emergent adverse events (TEAEs) were similar in both groups. Evaluating the role of darolutamide + ADT without docetaxel may provide new treatment options for patients with mHSPC.
What is the trial design?
ARANOTE is a phase III trial of mHSPC by conventional imaging, randomizing patients 2:1 to darolutamide 600 mg twice daily + ADT versus placebo + ADT. The primary endpoint was radiological progression-free survival (rPFS), and secondary endpoints included OS, time to initiation of subsequent anticancer therapy, time to CRPC, time to PSA progression, time to pain progression, and safety: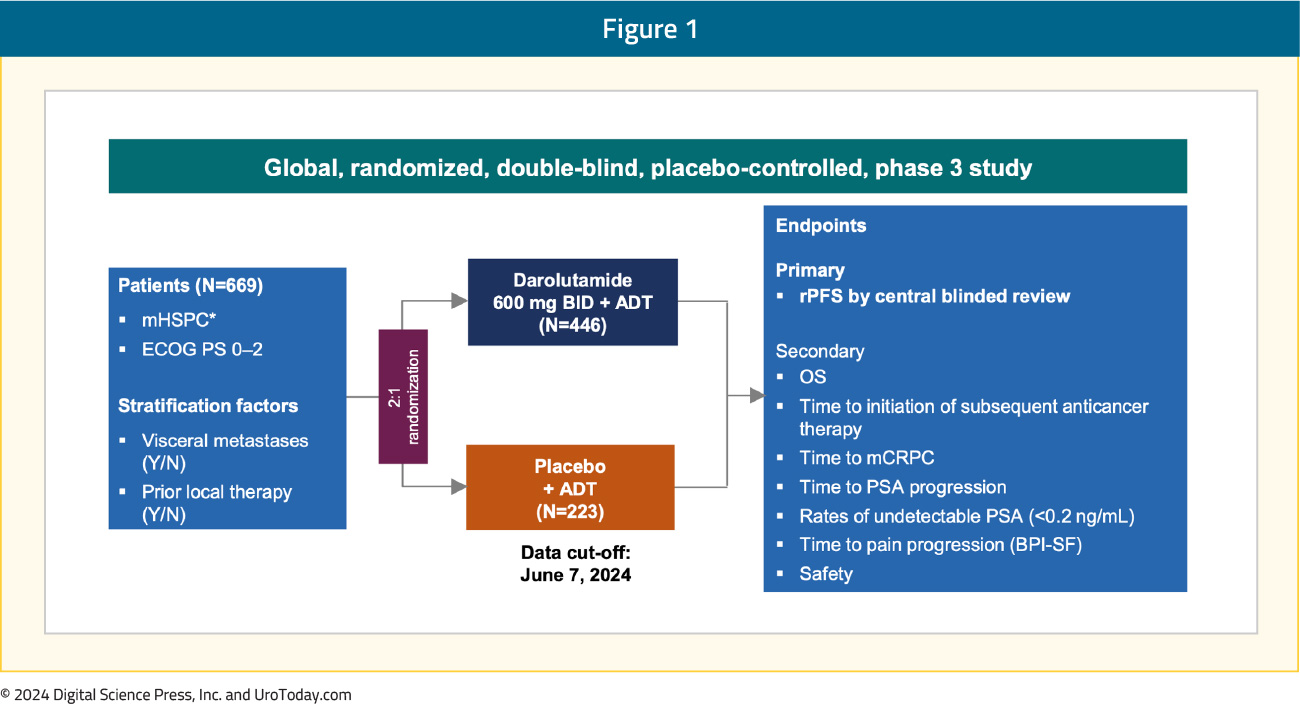
Darolutamide + ADT significantly improved rPFS versus placebo + ADT (HR 0.54, 95% CI 0.41–0.71):
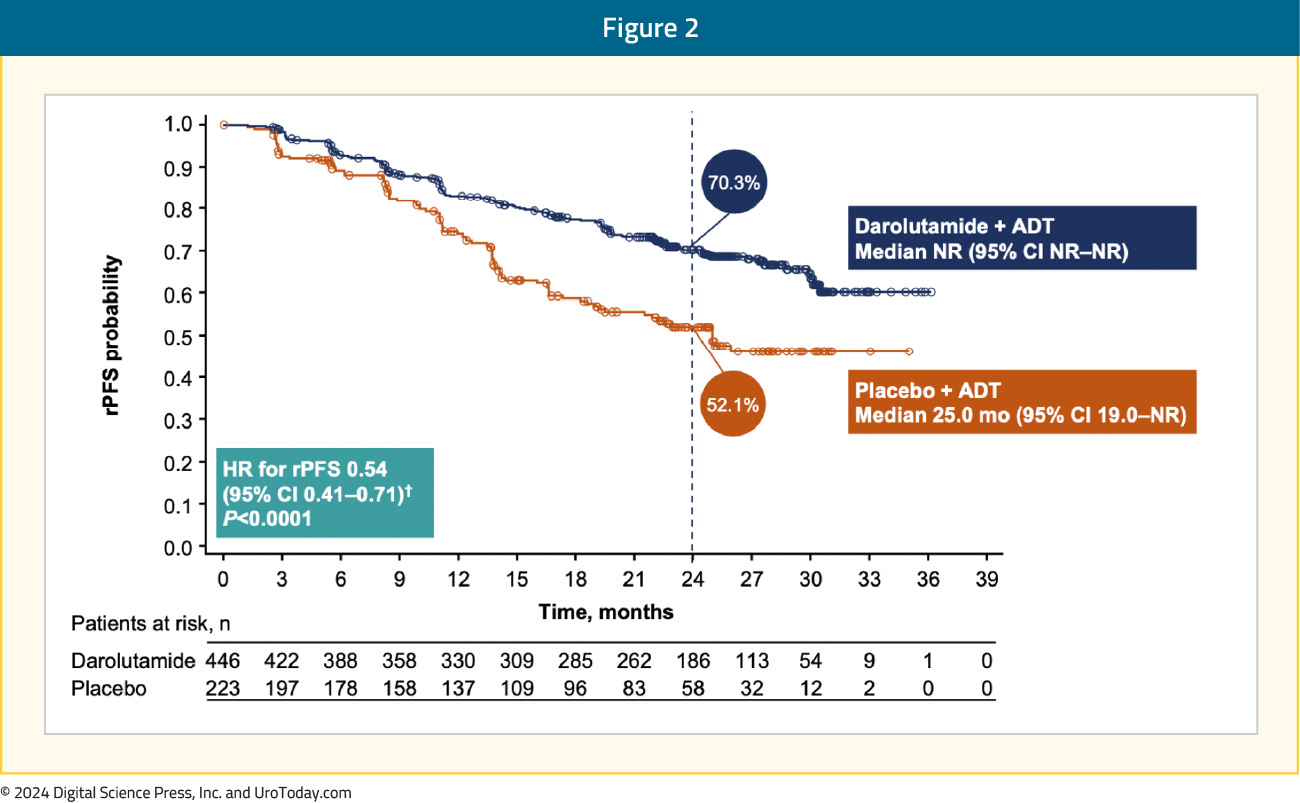
The rPFS benefit was consistent across subgroups, in particular for patients with low volume mHSPC:
Moreover, darolutamide was associated with a positive trend for OS (HR 0.81, 95% CI 0.59–1.12) and clinical benefits across all secondary efficacy endpoints, including time to CRPC (HR 0.40, 95% CI 0.32–0.51), time to PSA progression (HR 0.31, 95% CI 0.23–0.41), time to subsequent therapy (HR 0.40, 95% CI 0.29–0.56), and time to pain progression (HR 0.72, 95% CI 0.54–0.96):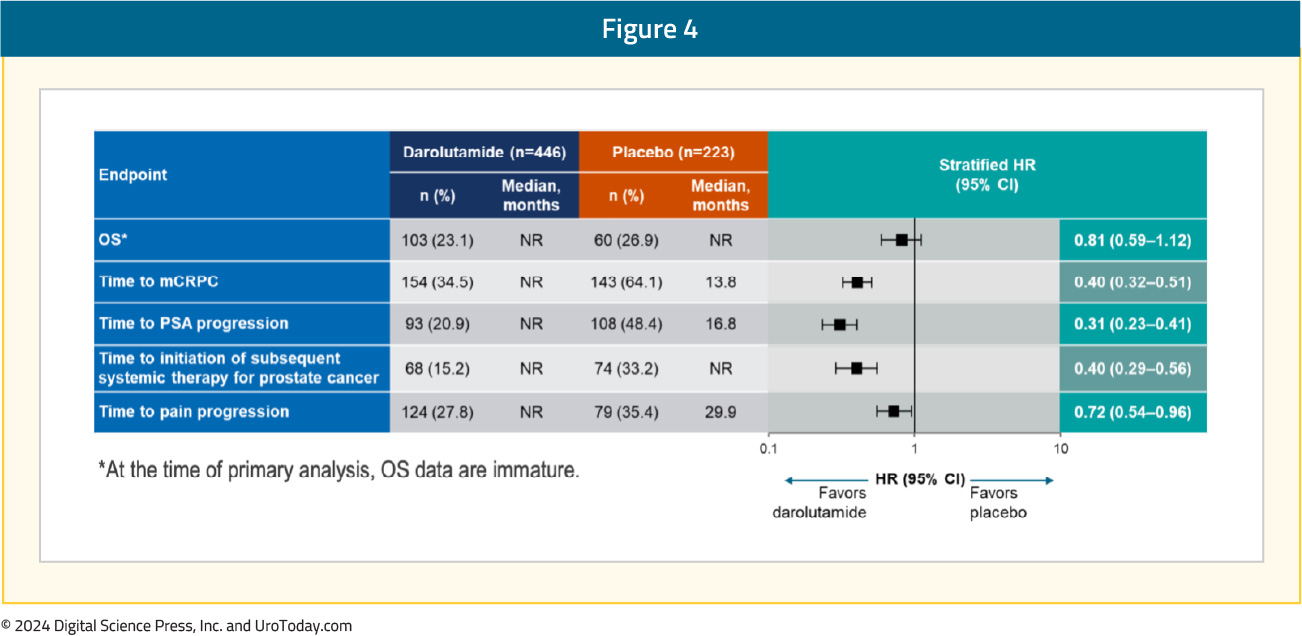
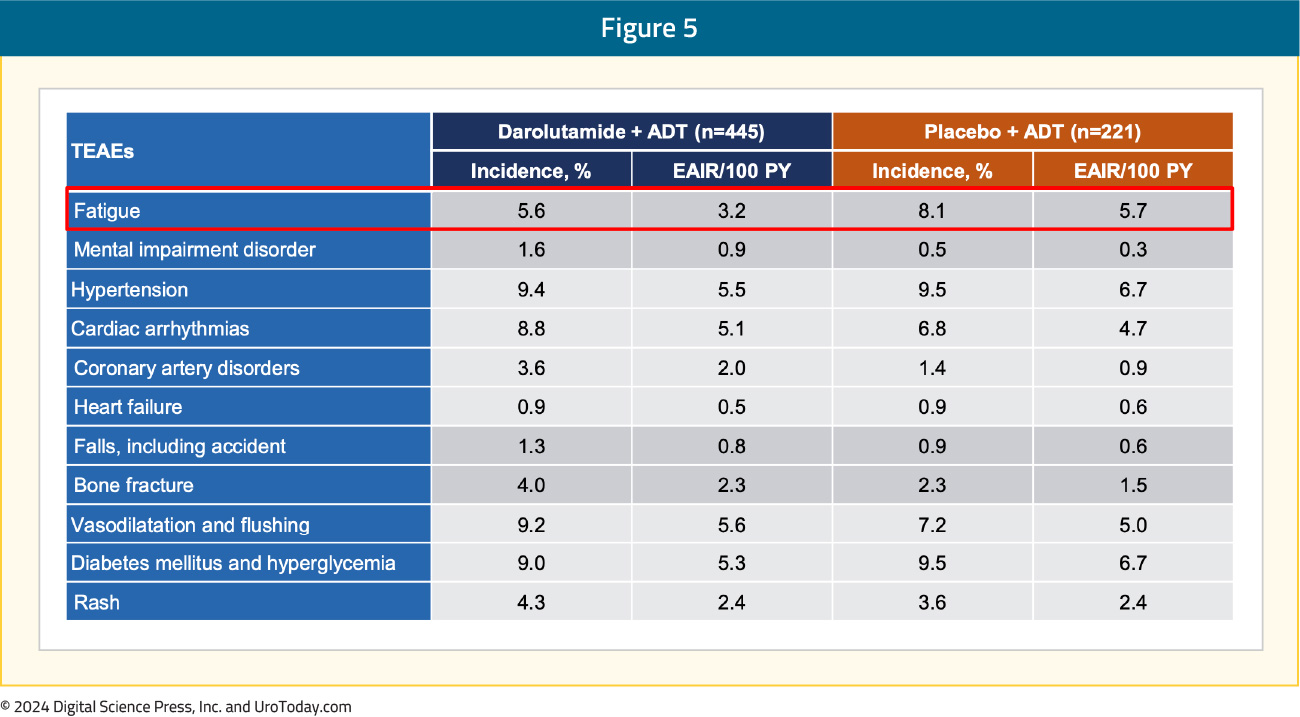
With regards to TEAEs of special interest, events were comparable between the two arms. Notably, there was less fatigue (5.6%) in the darolutamide + ADT arm compared to the placebo + ADT arm (8.1%):
What are the clinical implications?
These results from the second pivotal trial of darolutamide in patients with mHSPC add to the body of evidence from ARASENS and provide the option to select treatment in mHSPC, with or without docetaxel, to meet a patient’s individual needs and preferences. Particularly among patients with low volume mHSPC, darolutamide + ADT may be a reasonable treatment option.
PEACE-3
Why is this trial important?
Abiraterone2 and enzalutamide3 are standard of care options for first-line treatment of patients with mCRPC that have progressed on ADT. However, no combination thus far has been proven to increase both rPFS and OS in the first line mCRPC treatment setting.
What is the trial design?
PEACE-3 is a prospective phase III trial of mCRPC patients with bone metastases (no visceral metastases), who were either asymptomatic or mildly symptomatic. Patients were randomized 1:1 to radium-223 every 4 weeks for 6 cycles + enzalutamide versus enzalutamide alone. The primary endpoint was rPFS, and key secondary endpoints included safety and OS. Given the increased rate of skeletal fractures reported in the ERA-223 trial,4 a study amendment was introduced following the enrollment of the initial 119 patients to mandate the use of bone-protective agents:
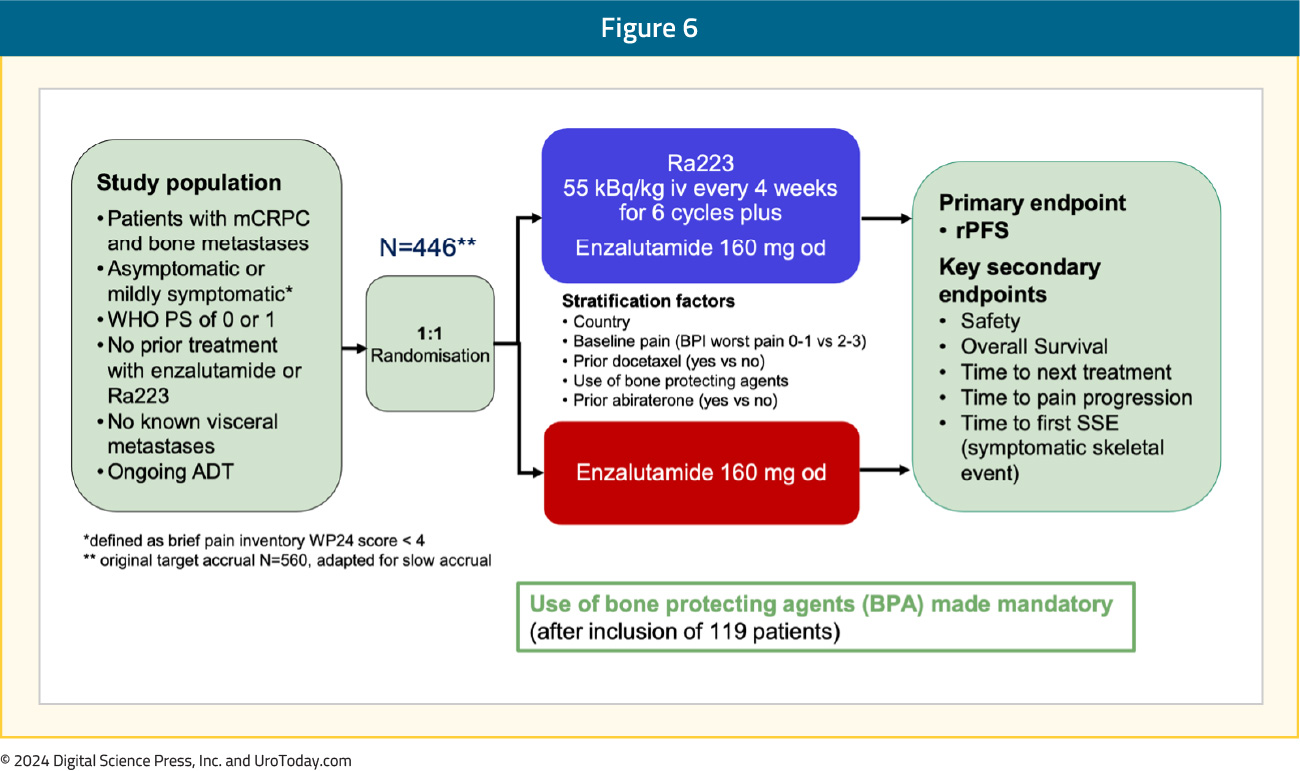
What were the key findings?
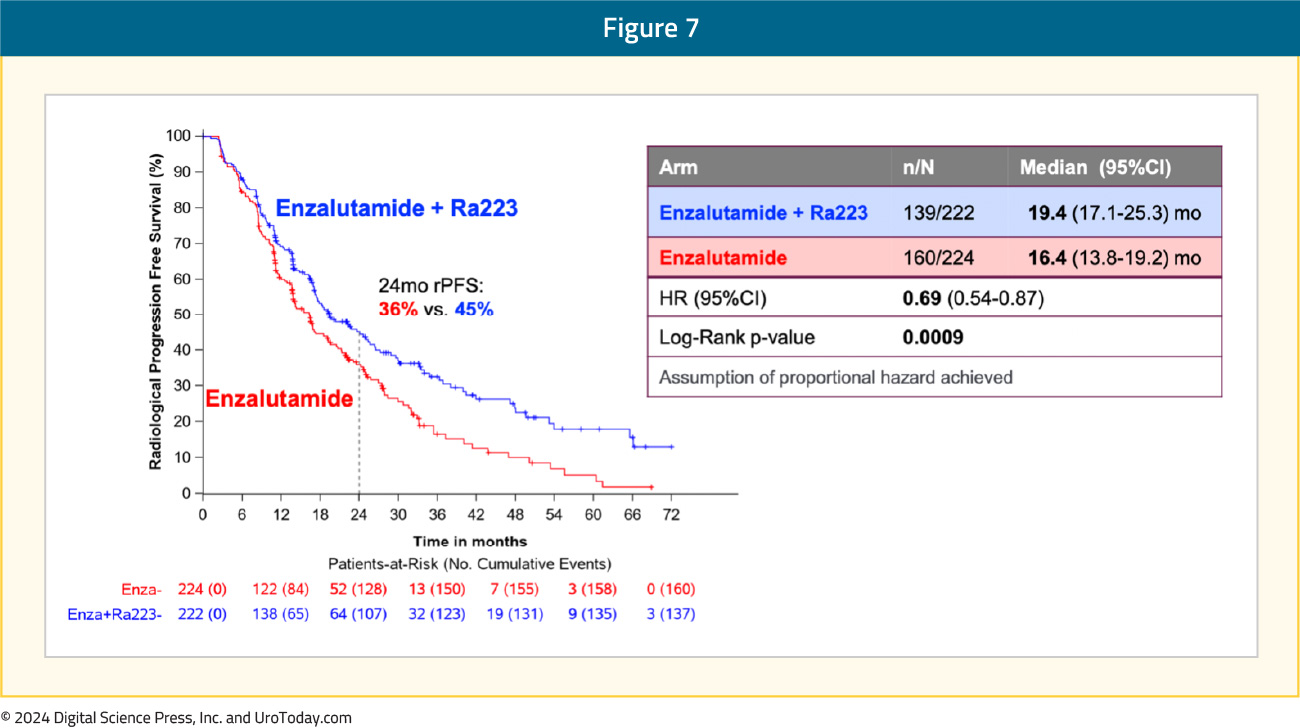
The addition of radium-223 to enzalutamide was associated with a significant improvement in rPFS (median: 19.4 versus 16.4 months; HR 0.69, 95% CI 0.54–0.87):
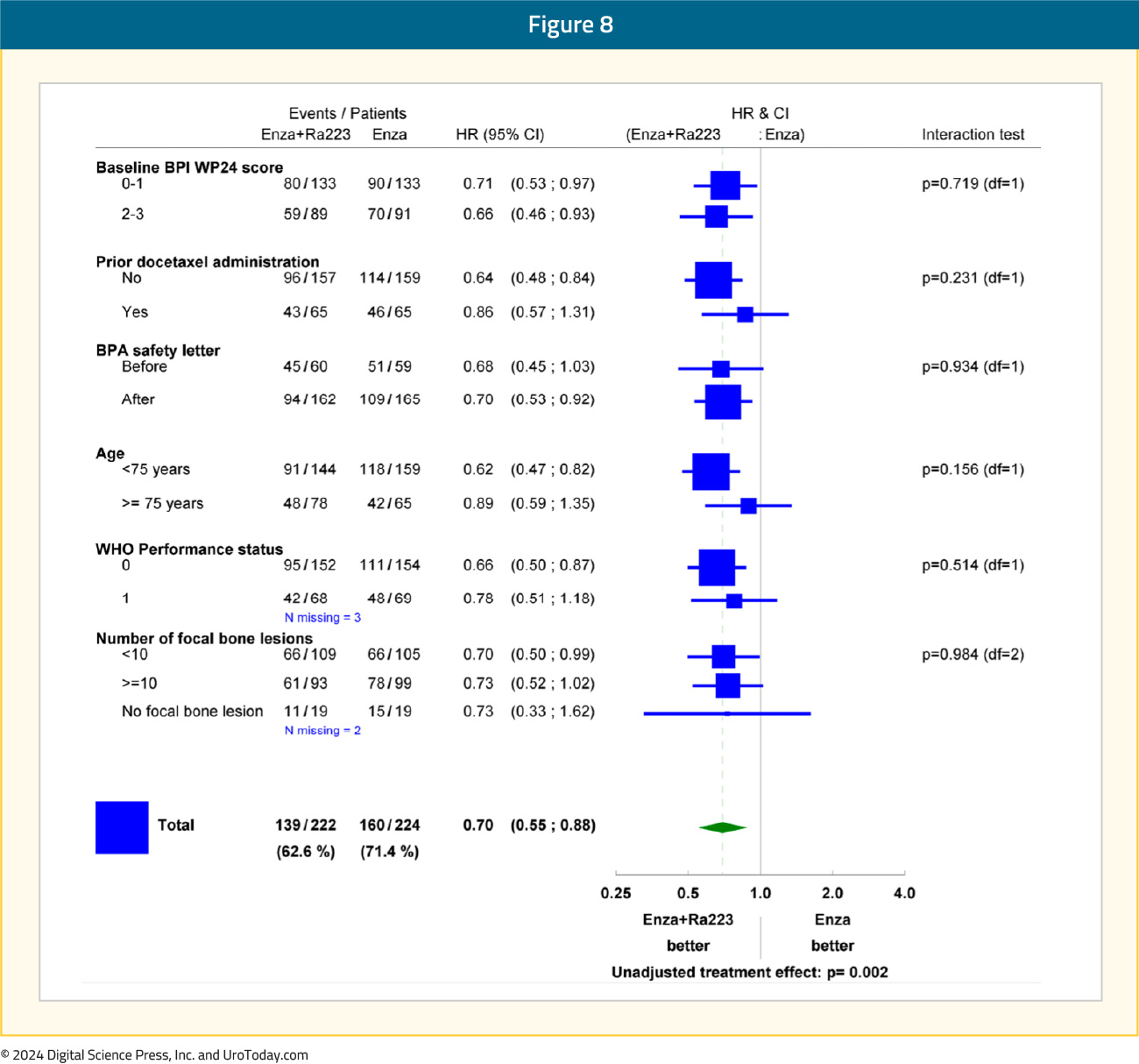
Subgroup analyses demonstrated consistent rPFS benefit in favor of radium-223 + enzalutamide:
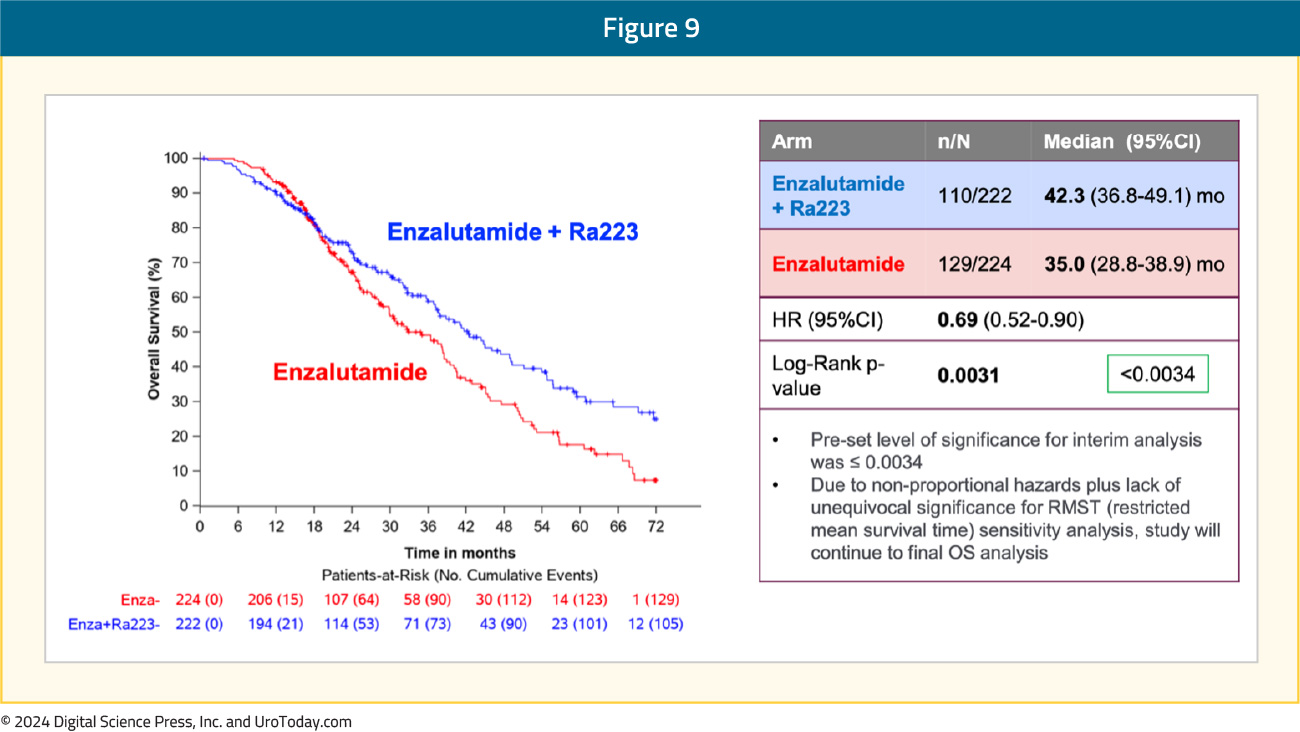
An OS benefit was also observed with radium-223 + enzalutamide: median OS of 42.3 months versus 35 months for enzalutamide monotherapy (HR 0.69, 95% CI 0.52–0.90, p=0.0031). While the p-value was below the pre-specified α threshold of 0.0034, there was evidence of non-proportional hazards. Given this, plus the lack of unequivocal significance for restricted mean survival time sensitivity analysis, the study will continue to the final overall survival analysis:
The most common grade ≥3 TEAEs in the radium-223 + enzalutamide arm were hypertension (34%), fatigue (5.5%), and fractures (5.1%):
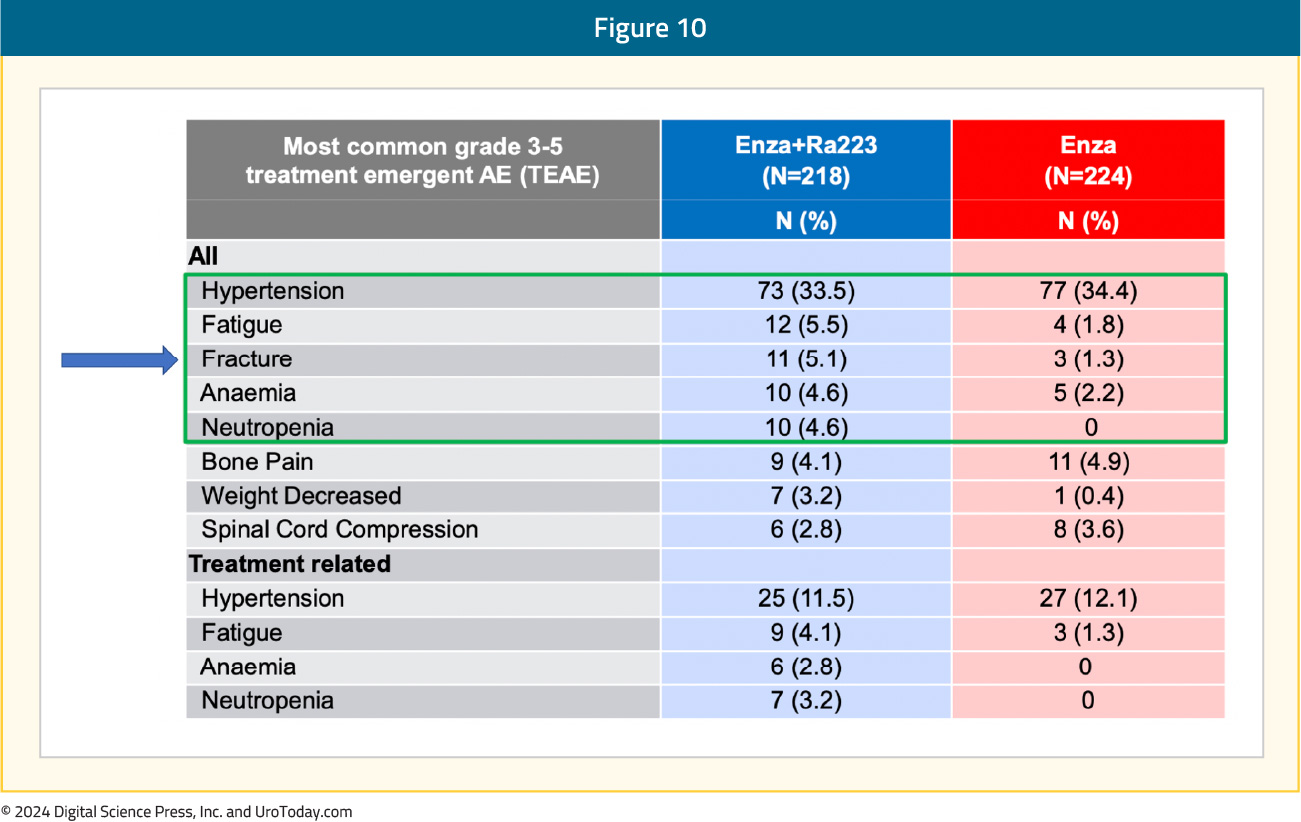
What are the clinical implications?
The results from PEACE-3 support the combination of radium-223 + enzalutamide in combination with a bone protective agent as a new first-line treatment option for mCRPC in patients who have not received a prior androgen receptor pathway inhibitor (ARPI). Patients receiving this combination should have no visceral metastases and asymptomatic/mildly symptomatic bone metastases.
SPLASH
Why is this trial important?
Based on the VISION trial,5 177Lu-PSMA-617 is approved post-docetaxel in the mCRPC setting based on an rPFS and OS benefit when added to standard of care in patients with PSMA-positive disease. 177Lu-PSMA-617 has also demonstrated a rPFS benefit in chemotherapy-naïve mCRPC patients (PSMAfore) but has not yet been approved in this setting. As such, additional radioligand agents in this disease space are needed.
What is the trial design?
SPLASH is a phase III, randomized trial evaluating 177Lu-PNT2002 in mCRPC patients experiencing disease progression following an ARPI. Patients were randomized 2:1 to 177Lu-PNT2002 every 8 weeks for 4 cycles versus the alternative ARPI (enzalutamide or abiraterone). The primary endpoint was rPFS, and key secondary endpoints included OS and safety:

What were the key findings?
Over a median follow-up of ~12 months, the SPLASH trial met its primary endpoint of a rPFS benefit with 177Lu-PNT2002 (median: 9.5 versus 6 months; HR: 0.71, 95% CI: 0.55–0.92):
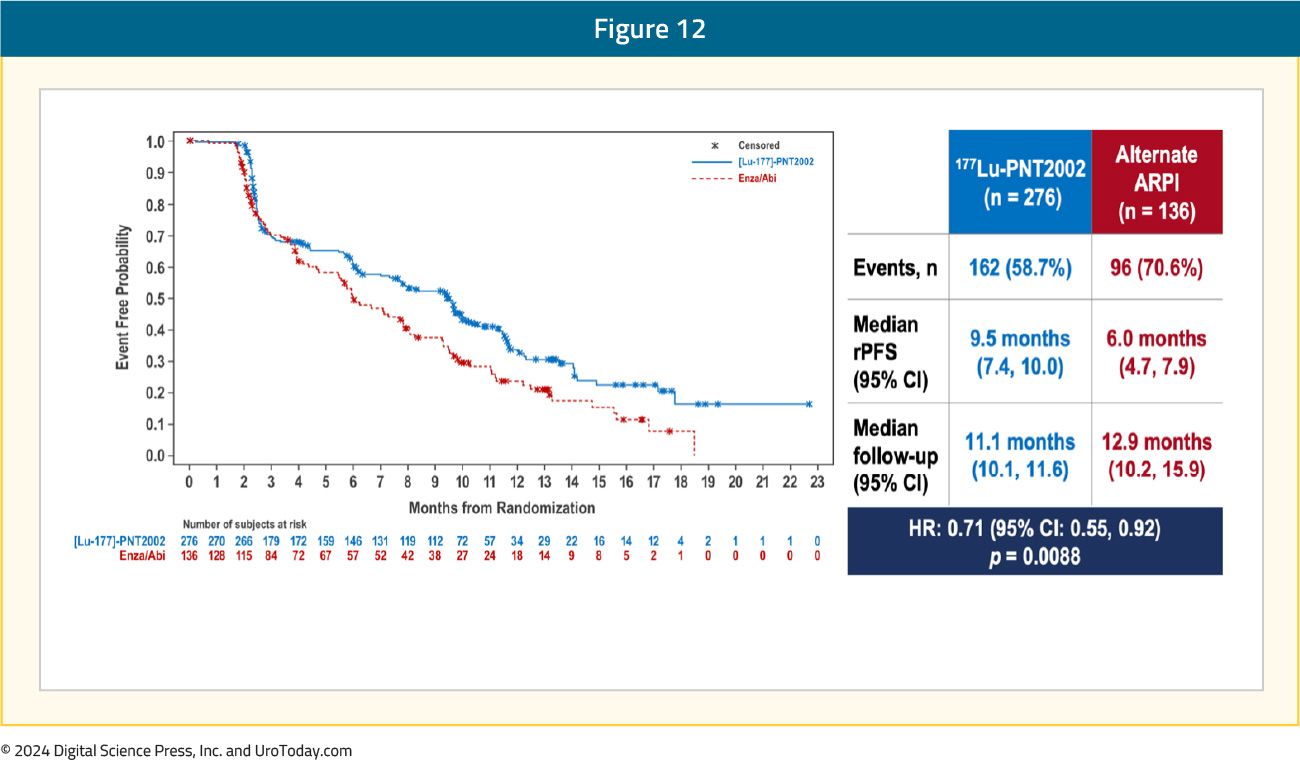
Subgroup analyses demonstrated consistent rPFS benefits with 177Lu-PNT2002 across the evaluated characteristics:
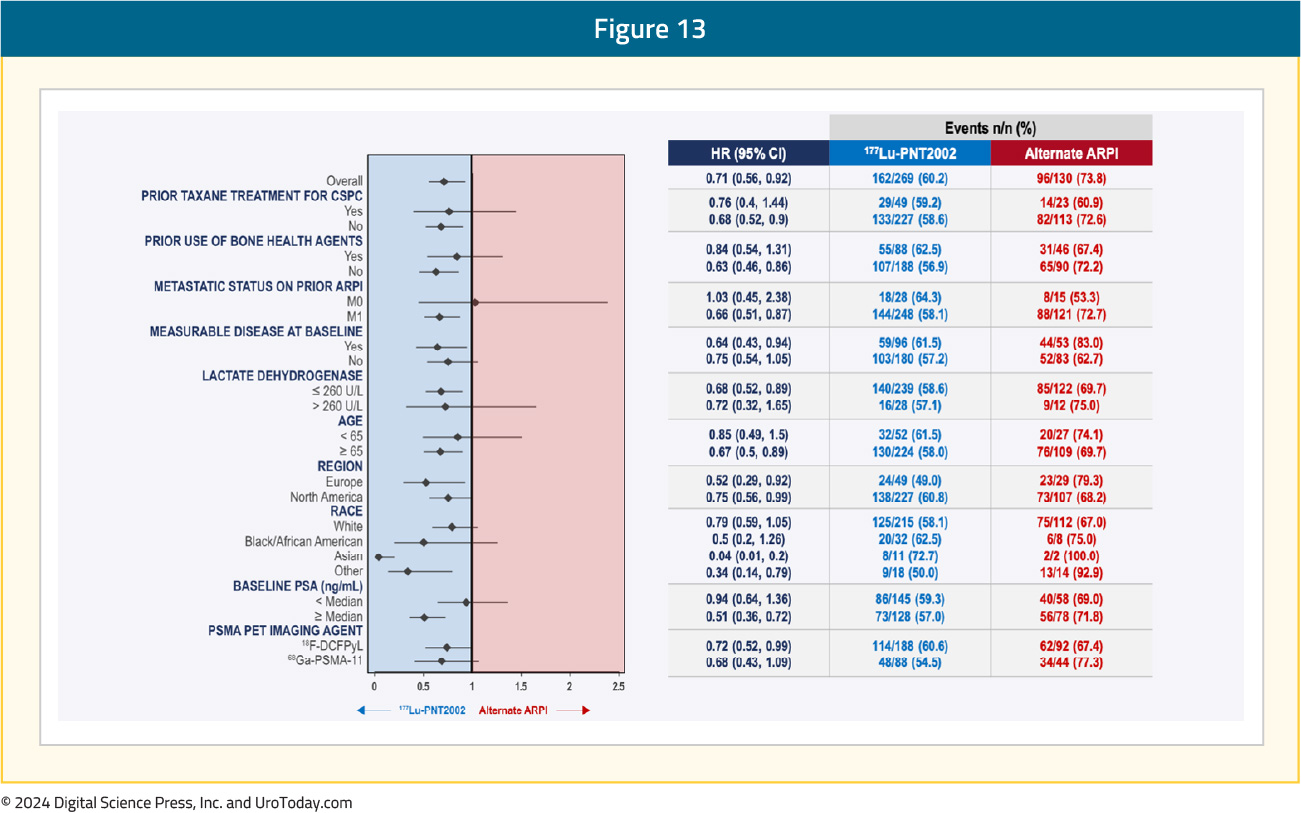
A complete response was observed in 9.3% of patients in the 177Lu-PNT2002 arm, compared to none in the control arm. The best overall response was observed in 38% and 12% of patients, respectively (p=0.0021), and the median durations of response were 9.4 and 7.3 months, respectively:
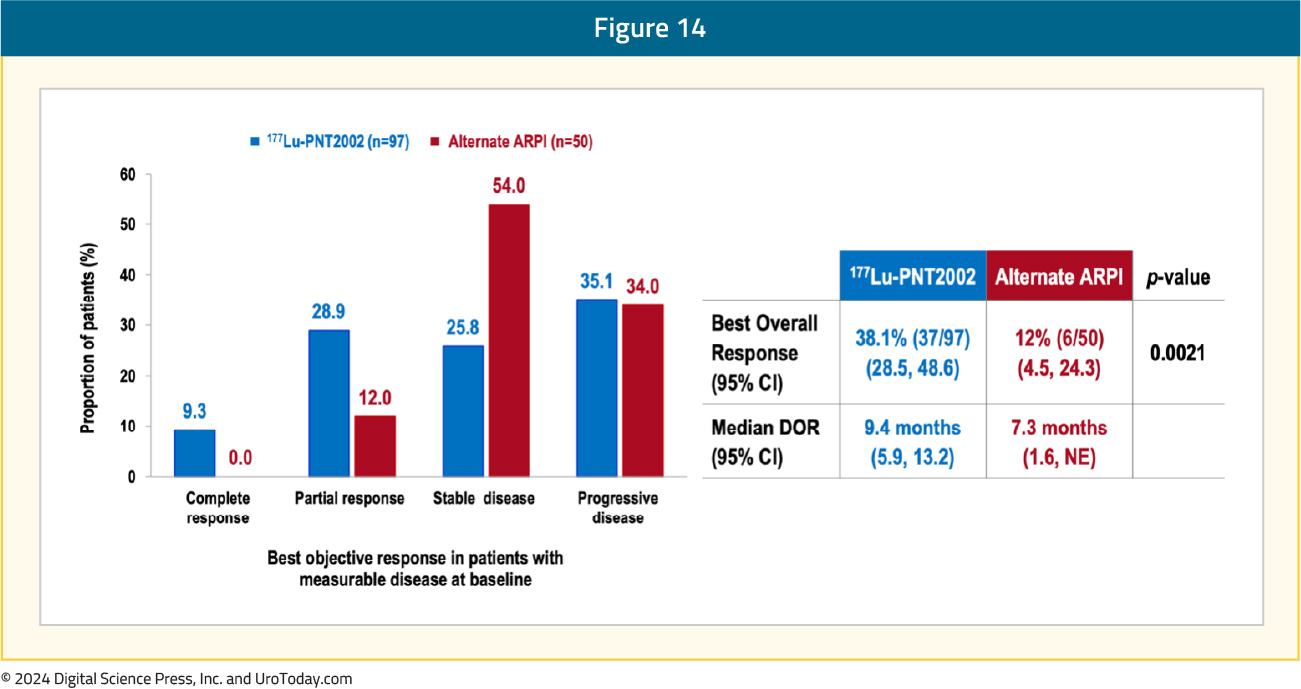
The first interim analysis of OS did not demonstrate a benefit for 177Lu-PNT2002 (median: 20.8 months versus NE; HR 1.11, 95% CI 0.73–1.69), likely secondary to significant cross-over in the alternate ARPI arm: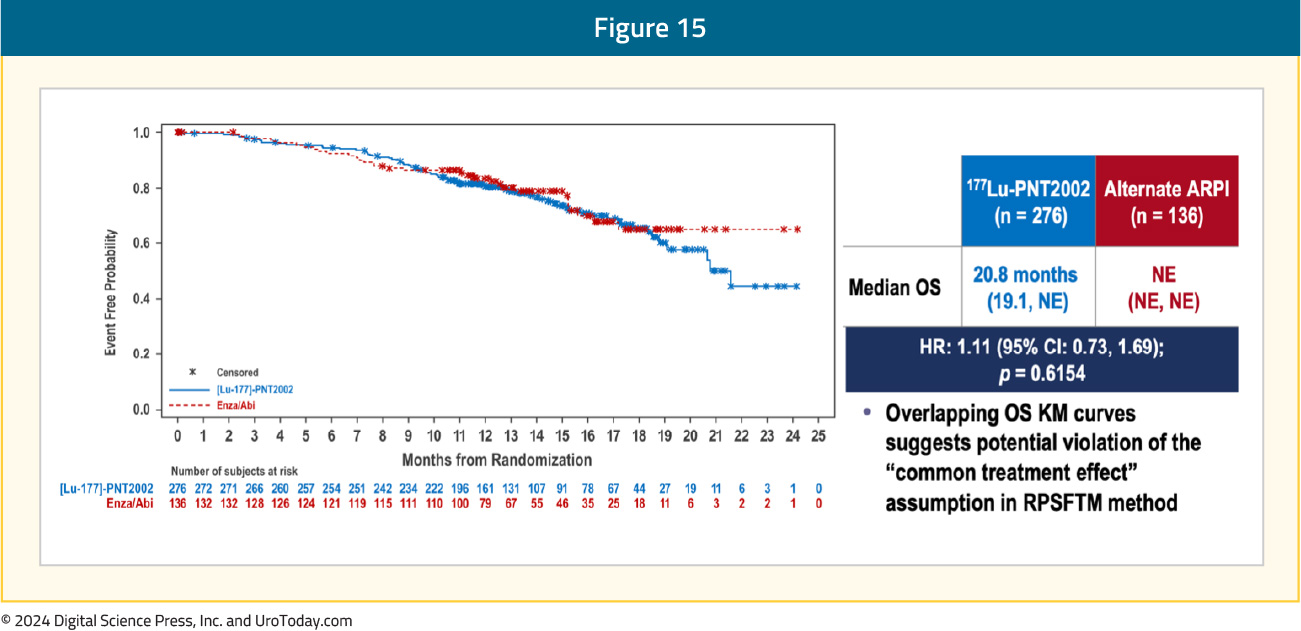
The adverse event profile favored 177Lu-PNT2002 – grade ≥3 events were observed in 10% and 12% of patients in the experimental and control arms, respectively. There were no TEAEs leading to death, and TEAEs leading to treatment discontinuation were observed in 2% and 6% of patients, respectively:

What are the clinical implications?
The SPLASH trial demonstrated that 177Lu-PNT2002 may provide a second radioligand therapy option for patients in the pre-chemotherapy (after an ARPI) mCRPC disease space, with only 4 cycles of treatment.
Published October 2024

