ESMO 2024 Quick Take Insights: A Focus on PEACE-3
The ESMO 2024 annual meeting in Barcelona, Spain, featured many practice-changing trials and hypothesis-generating data in genitourinary cancer. One of the most highly anticipated trials in the metastatic castration-resistant prostate cancer (mCRPC) disease space was the phase III PEACE-3 trial – the focus of this ESMO 2024 Quick Take Insights.
Why is this trial important?Abiraterone1 and enzalutamide2 are standard of care options for first-line treatment of patients with mCRPC that have progressed on ADT. However, no combination thus far has been proven to increase both radiographic progression-free survival (rPFS) and overall survival (OS) in the first line mCRPC treatment setting. Moreover, radium-223, based on the phase 3 ALSYMPCA trial,3 has been approved for symptomatic bone mCRPC for over a decade. In ALSYMPCA, OS among patients receiving radium-223 (median OS 14.9 months) significantly improved overall survival compared to placebo (median OS 11.3 months; HR 0.70, 95% CI 0.58 to 0.83):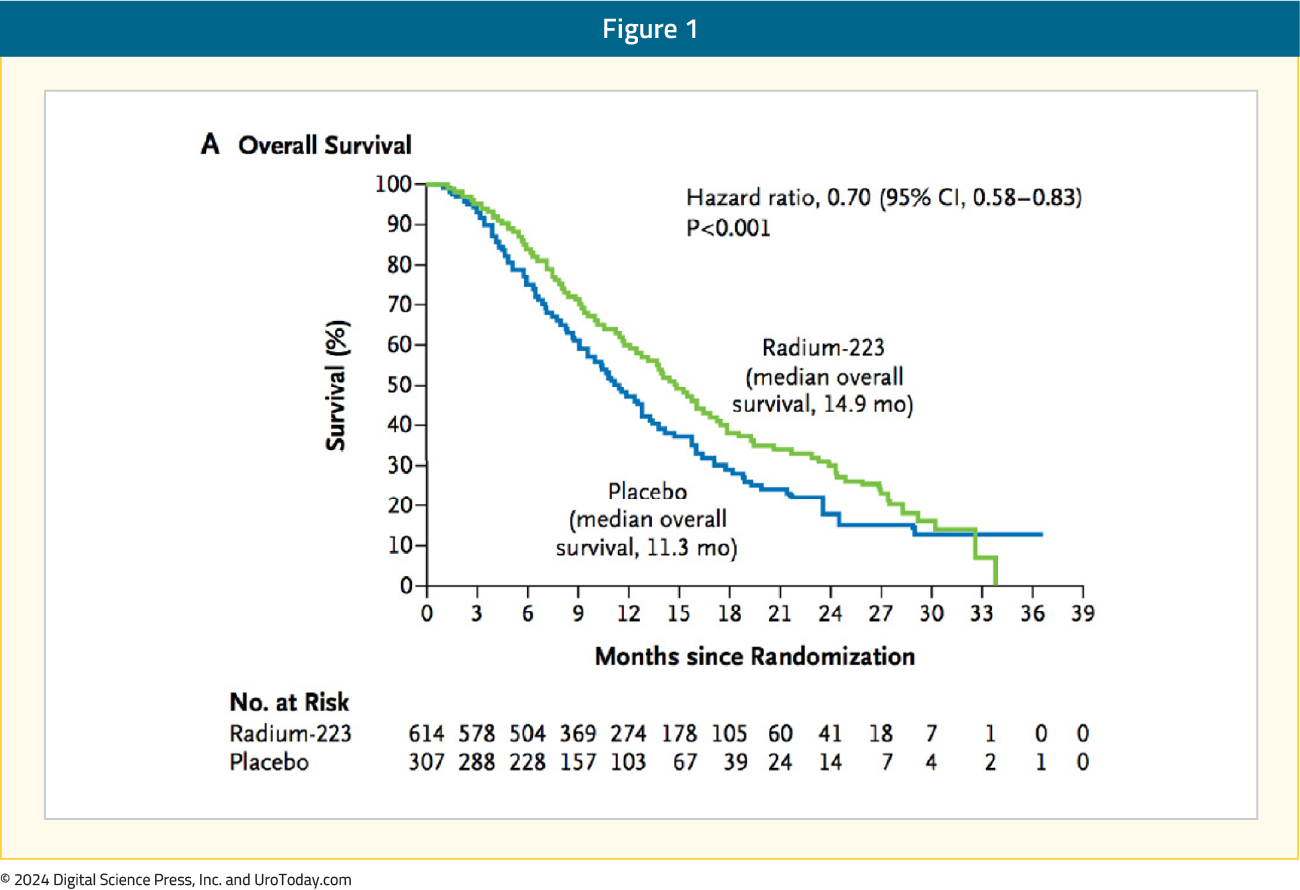 Although there is proven efficacy for radium-223 in mCRPC, utilization in recent years has been limited. There are likely several reasons for this lack of use, including (i) difficulty with interpreting the positive ALSYMPCA trial in the context of an immensely changed therapeutic landscape from 2008 to 2024; (ii) radium-223 targets activated bone stromal lesions but does not target tumors in soft tissue. As such, better/broader patient selection is needed for radium-223, which has previously been demonstrated in the RALU study of patients receiving sequential radium-223 and 177Lu-PSMA-617.4
Although there is proven efficacy for radium-223 in mCRPC, utilization in recent years has been limited. There are likely several reasons for this lack of use, including (i) difficulty with interpreting the positive ALSYMPCA trial in the context of an immensely changed therapeutic landscape from 2008 to 2024; (ii) radium-223 targets activated bone stromal lesions but does not target tumors in soft tissue. As such, better/broader patient selection is needed for radium-223, which has previously been demonstrated in the RALU study of patients receiving sequential radium-223 and 177Lu-PSMA-617.4
PEACE-3 is a prospective phase III trial of mCRPC patients with bone metastases (no visceral metastases), who were either asymptomatic or mildly symptomatic and had not been previously exposed to enzalutamide or radium-223. Eligible patients were randomized 1:1 to radium-223 every 4 weeks for 6 cycles + enzalutamide versus enzalutamide alone. The primary endpoint was rPFS, and key secondary endpoints included safety and OS. Given the increased rate of skeletal fractures reported in the ERA-223 trial,5 a study amendment was introduced following the enrollment of the initial 119 patients to mandate the use of bone-protective agents: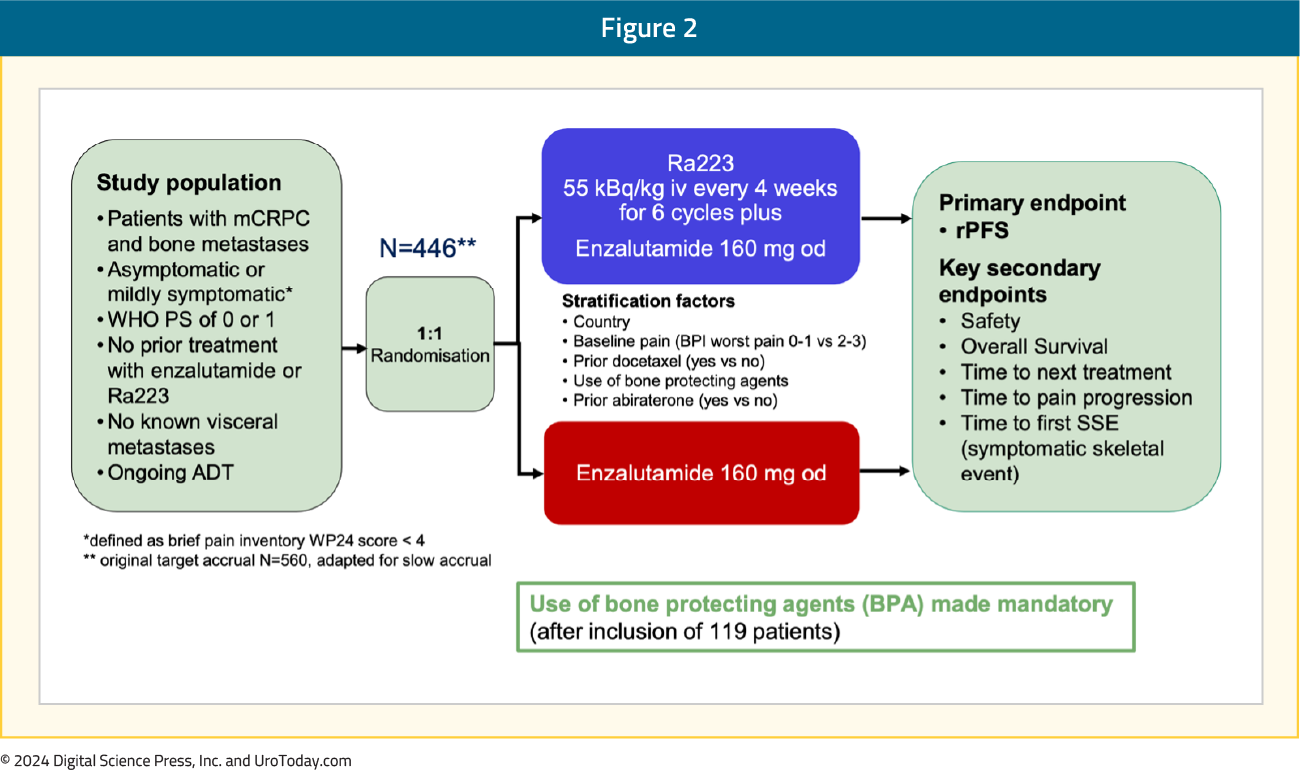 What were the key findings?
What were the key findings?
The addition of radium-223 to enzalutamide was associated with a significant improvement in rPFS (median: 19.4 versus 16.4 months; HR 0.69, 95% CI 0.54–0.87):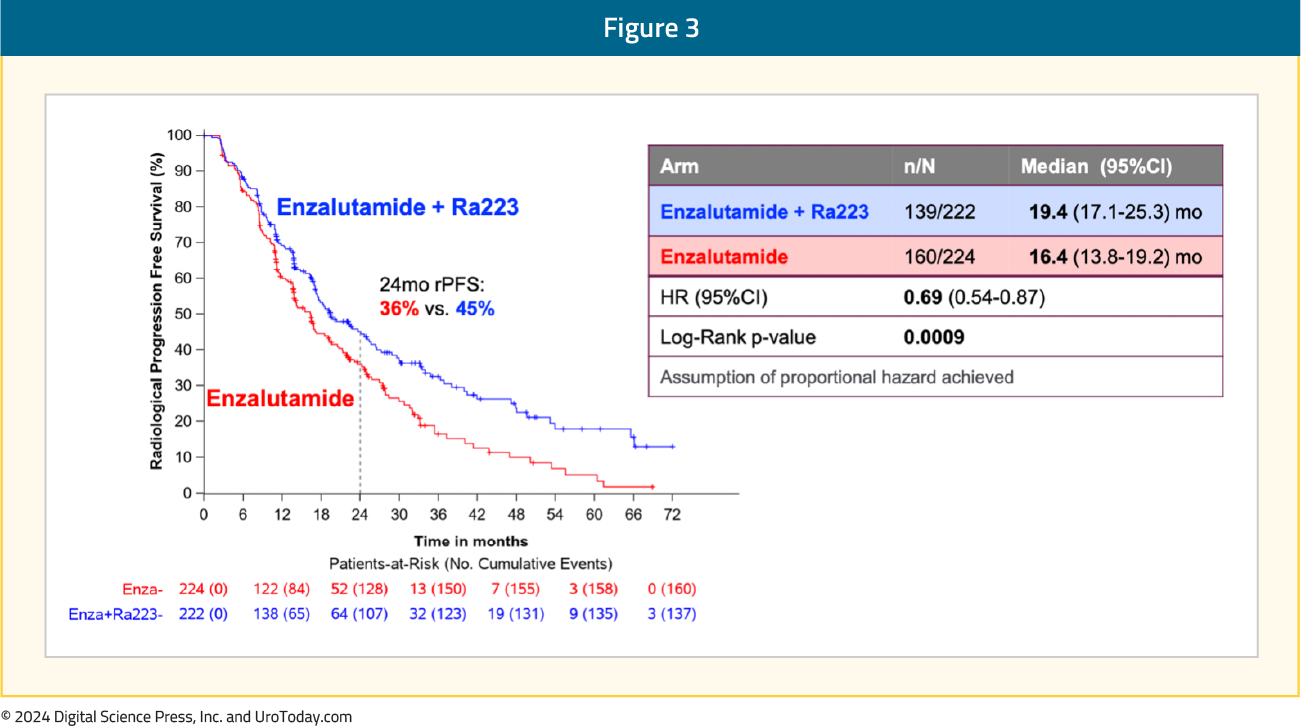 Subgroup analyses demonstrated consistent rPFS benefit in favor of radium-223 + enzalutamide:
Subgroup analyses demonstrated consistent rPFS benefit in favor of radium-223 + enzalutamide: An OS benefit was also observed with radium-223 + enzalutamide: median OS of 42.3 months versus 35 months for enzalutamide monotherapy (HR 0.69, 95% CI 0.52–0.90, p=0.0031). While the p-value was below the pre-specified α threshold of 0.0034, there was evidence of non-proportional hazards. Given this, plus the lack of unequivocal significance for restricted mean survival time sensitivity analysis, the study will continue to the final overall survival analysis:
An OS benefit was also observed with radium-223 + enzalutamide: median OS of 42.3 months versus 35 months for enzalutamide monotherapy (HR 0.69, 95% CI 0.52–0.90, p=0.0031). While the p-value was below the pre-specified α threshold of 0.0034, there was evidence of non-proportional hazards. Given this, plus the lack of unequivocal significance for restricted mean survival time sensitivity analysis, the study will continue to the final overall survival analysis: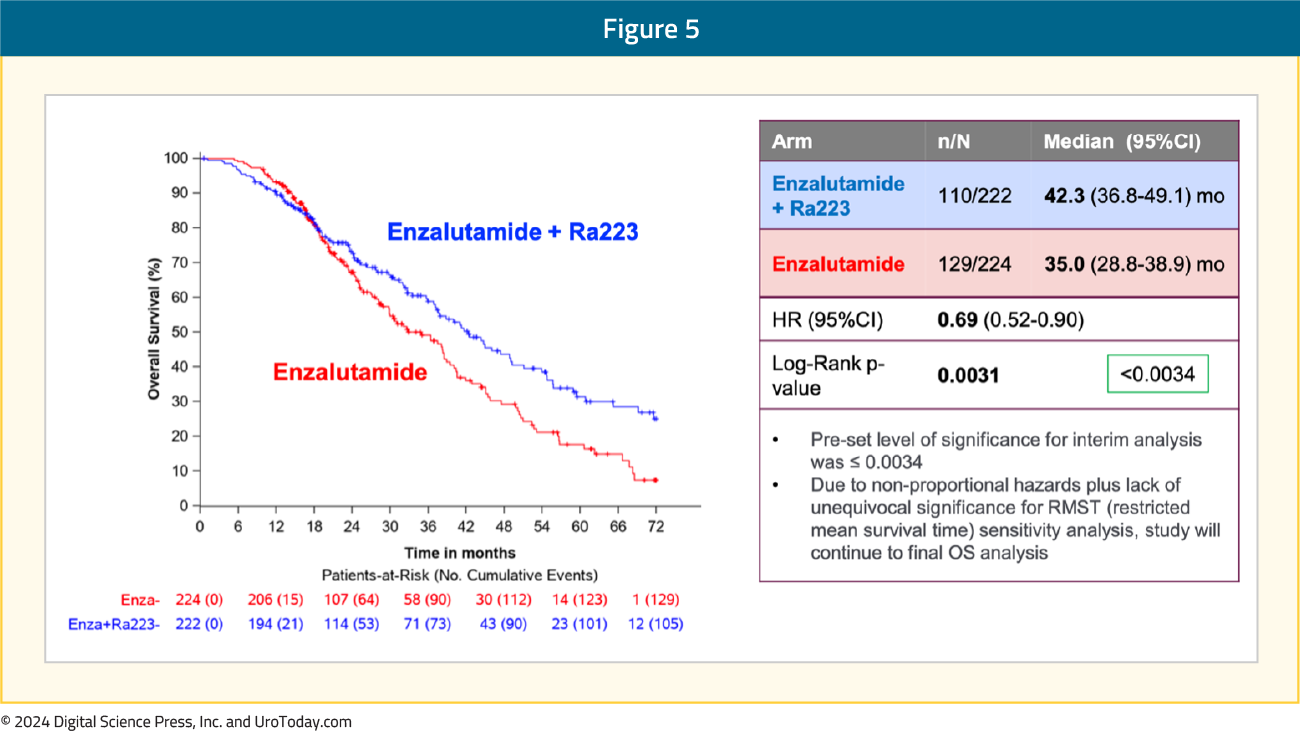 The most common grade ≥3 TEAEs in the radium-223 + enzalutamide arm was hypertension (34%), fatigue (5.5%), and fractures (5.1%):
The most common grade ≥3 TEAEs in the radium-223 + enzalutamide arm was hypertension (34%), fatigue (5.5%), and fractures (5.1%):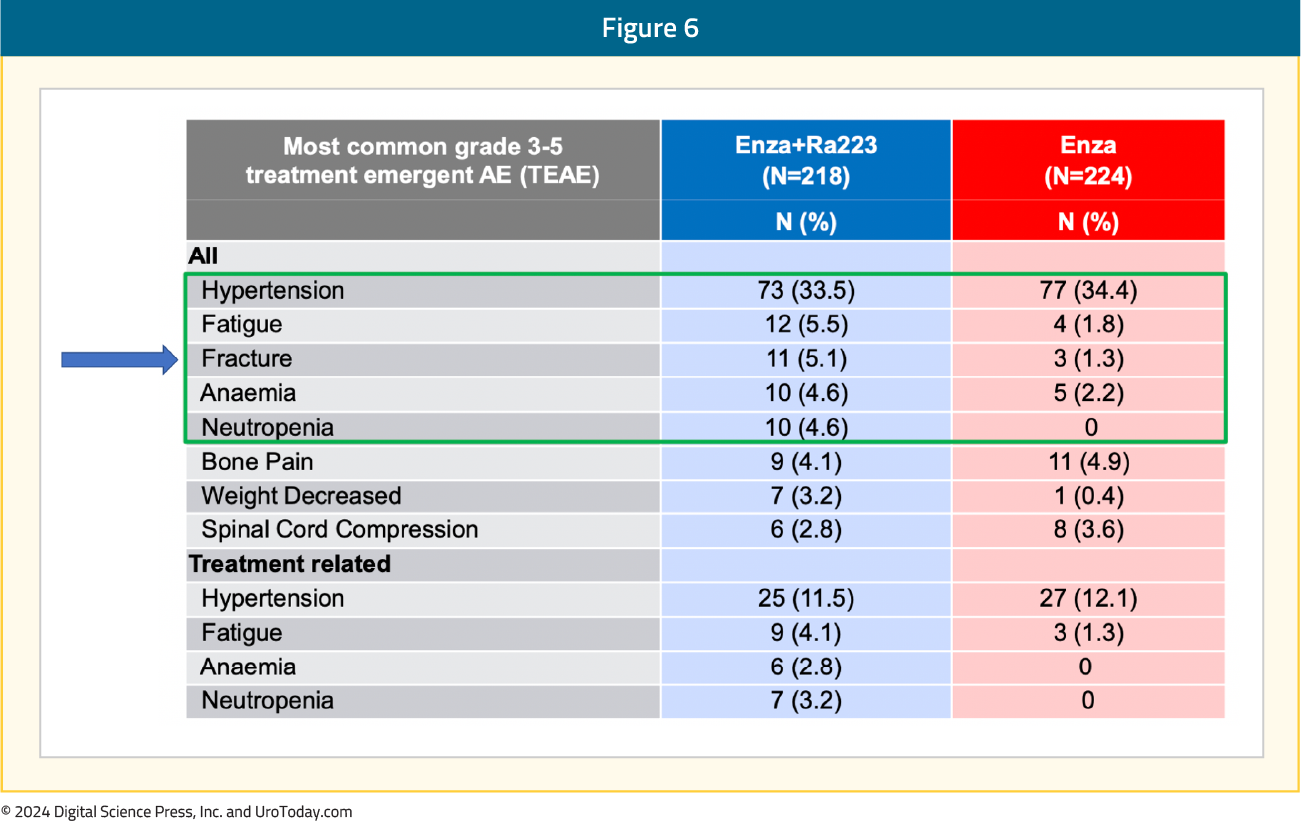 What are the clinical implications?
What are the clinical implications?
The results from PEACE-3 support the combination of radium-223 + enzalutamide in combination with a bone protective agent as a new first-line treatment option for mCRPC in patients who have not received a prior androgen receptor pathway inhibitor (ARPI). These results potentially elevate six cycles of radium-223 into the first line mCRPC disease space in combination with enzalutamide, with the caveat that patients being considered for this combination must have no evidence of visceral metastases and have asymptomatic/mildly symptomatic bone metastases (as opposed to ALSYMPCA where patients had symptomatic bone metastases). Moreover, the results of ERA-223 and those from PEACE-3 highlight the absolute necessity of using bone protective agents in mCRPC patients, specifically for patients receiving radium-223 + enzalutamide combination therapy.
Published October 2024
