(UroToday.com) The 2024 South Central AUA annual meeting included a session on bladder cancer, featuring a presentation by Dr. Danica May discussing whether there is an end to the BCG shortage in sight. Dr. May notes that BCG is utilized in high risk patients with CIS/high grade T1/high risk Ta urothelial carcinoma with a six week induction course. Importantly, we should avoid use in patients with low or intermediate risk disease:
- We should be using perioperative single dose chemotherapy for low or intermediate risk disease.
- There should be no induction for low risk disease.
- We should consider induction chemotherapy or immunotherapy for intermediate risk disease and consider maintenance.
Based on EORTC 30962, high risk patients who respond to induction therapy should get maintenance BCG for up to 3 years, if available. Additionally, if patients have recurrent Ta or CIS after a single induction course, we can consider repeat induction therapy. However, if patients have high grade T1 after induction therapy, these patients should be offered radical cystectomy. Dr. May notes that we should cease BCG use if patients are intolerant or have documented recurrence on TURBT of high grade NMIBC and/or CIS within 6 months of two induction courses or induction and maintenance.
At least 8 strains of BCG are used intravesically worldwide, with BCG TICE and Connaught most often used in the United States. However, the United States previously used Sanofi Pasteur Connaught strain (TheraCys/ImmuCyst), which suspended production in 2012, permanently closed in 2017, and led to a global shortage. Subsequently, this led to a monopoly, with Merck as the only producer in the United States (TICE BCG), resulting in rationing and shortages. Part of this issue is that BCG takes 3 months to grow.
Prior to the shutdown, Merck supplied 28% of the US BCG stock. They were able to subsequently ramp up production by more than 100%, as well as decreasing lead time for production by 40%. In 2016, manufacturing at full extent, led to 600,000 - 870,000 vials produced annually. In October 2020, Merck started construction of a new manufacturing facility in Durham, NC, with a completion date of 2025-2026, and the goal to triple production.
Dr. May then discussed treatment options in the setting of limited BCG:
- Dose reduce to 1/2 or 1/3 dose as needed for high risk NMIBC patients
- Maintenance BCG for only 1 year
- BCG naïve patients with high risk disease should be prioritized for induction BCG over maintenance
- Gemcitabine, epirubicin, docetaxel, valrubicin, mitomycin, or sequential gemcitabine + docetaxel or gemcitabine + mitomycin may also be considered with an induction and possible maintenance regimen
- Patients with high risk features who are not willing to take any potential oncologic risks with alternative agents, should be offered initial radical cystectomy
The following are options highlighted by the NCCN for BCG unresponsive or BCG intolerant patients: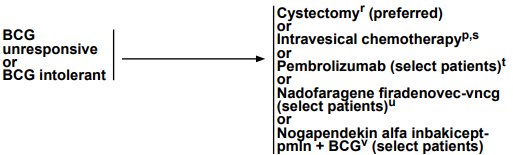
There are relevant clinical trials ongoing with checkpoint inhibitors in combination with BCG trials:
- S1602: closed to enrollment, TICE versus Tokyo-172 with intradermal injection, with results expected in December 2024
- EA8212/BRIDGE: gemcitabine + docetaxel versus BCG non-inferiority trial, with a goal of 870 patients and currently enrolling
- SunRISE-3: a phase 3 study of PD-1 inhibitor cetrelimab + TAR-200 versus TAR-200 alone for BCG-naïve patients with high risk NMIBC
- PIVOT-006: an intermediate risk trial of cretostimogene grenadenorepvec (CG0070) after TURBT versus TURBT alone, which is currently enrolling
Dr. May concluded her presentation discussing whether there is an end to the BCG shortage in sight with the following take-home points:
- BCG has been the standard of care for 40 years of high risk NMIBC
- New data and trials will hopefully open up new treatments for these patients in the near future
Presented by: Danica May, MD, Urologic Oncologist, The University of Kansas Medical School, Kansas City, KS
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 South Central American Urological Association (AUA) Annual Meeting, Colorado Springs, CO, Wed, Oct 30 – Sat, Nov 2, 2024.

