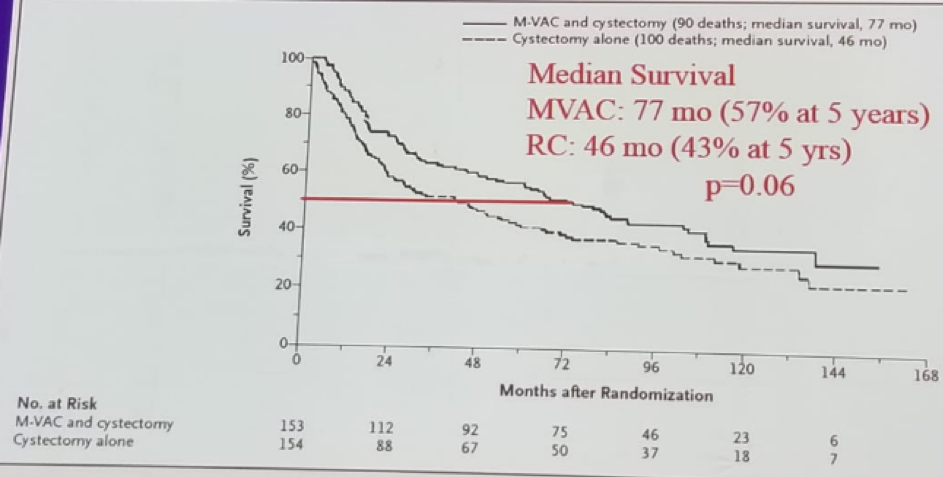
The original paper published supporting the use of neoadjuvant chemotherapy (NAC) for MIBC patients was a prospective randomized trial comparing survival in patients treated with NAC (MVAC) followed by RC vs. RC alone.1 Overall 317 patients with clinical stage T2-T4 N0M0 were included. Median survival was 77 months (57% at 5 years) in the MVAC arm, compared to 46 months (43% at 5 years) (Figure 1). Furthermore, the pathological T0 rate after cystectomy was 38% at the MVAC group compared to 15% in the RC group. It was concluded that NAC increases overall survival (OS) and disease-specific survival (DSS) over RC alone.
Figure 1 – Median survival among patients who received MVAC+ RC vs. those who received RC alone
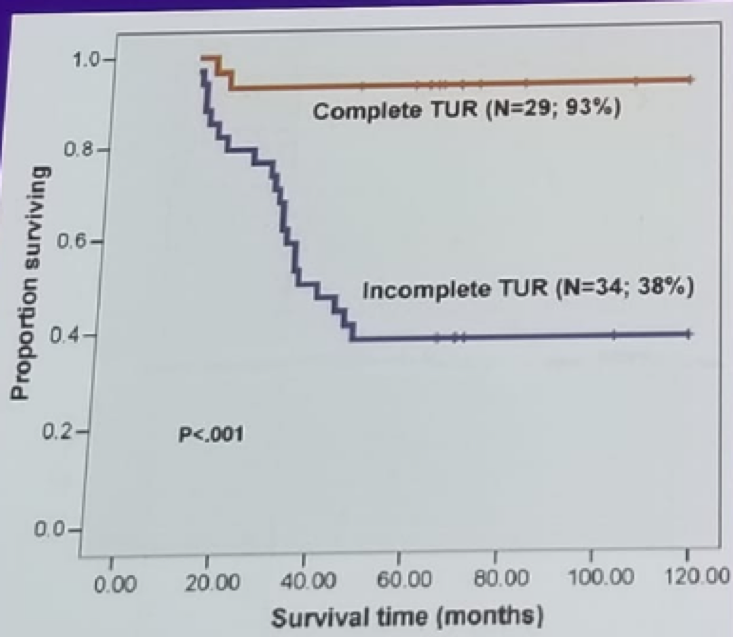
Another interesting study analyzed the outcome of patients who refused RC after NAC for MIBC. Overall 63 patients were analyzed and the primary outcome was OS.2 Multivariable analysis demonstrated that factors affecting survival included number of tumors, tumors above 5 cm, incomplete restaging TUR, and invasive stage at relapse. The OS rate of patients not undergoing salvage cystectomy was 54% at 5 years. Complete restaging TUR was a very significant predictor of survival (Figure 2). In conclusion, of patients who completely respond to NAC, approximately 50% survived with their bladders at 10 years. Non-invasive recurrence can be managed with TUR and intravesical chemotherapy. If salvage cystectomy is done after a recurrence, less than half survive.
Figure 2: Overall survival stratified by completeness of restaging TURBT:
The question of whether survival equivalent to immediate postchemotherapy RC can be achieved, if the bladder is preserved in patients achieving a complete response to NAC, remains unanswered. We need to be able to predict those patients likely to achieve a post-chemotherapy pathological stage of T0. Molecular subtypes in MIBC will have a substantial role in the future of predicting response and survival after NAC. The 4 known molecular subtypes have been analyzed and their response to NAC compared. Using a genomic subtyping classifier (GSC), it was possible to predict consensus subtypes with highest clinical impact in the context of NAC:
- Luminal tumors had the best OS with and without NAC
- Claudin-low tumors were associated with poor OS irrespective of treatment regimen
- Basal tumors showed the highest improvement in OS with NAC compared to surgery alone
Tumors exhibiting mutations in genes associated with DNA damage repair (DDR) such as ERCC2, ERBB2, ATM, RB1, or those grouped into the basal/squamous subtype display a greater degree of cisplatin sensitivity as demonstrated by more favorable clinical outcomes among these patients. In contrast, the luminal-papillary and luminal-infiltrated subtypes show poor response to NAC, and these patients may be the best candidates for immediate RC, without NAC.
Tumors with upregulation of the immune checkpoint markers (luminal-infiltrated and basal/squamous) may benefit from neoadjuvant or adjuvant treatment with anti-PDL1, PD1, and/or CTLA-4 immunotherapy. However, prospective trials are desperately required.
Lastly, Dr. Benson discussed the bladder preservation option. The rationale for this option include the fact that there is less surgery, no need for urinary diversion, preservation of sexual function in both men and women, and overall better quality of life. The proposed protocol of bladder preservation includes:
- Clinical staging (CT scan and TURBT)
- NAC MVAC (3 cycles)
- Clinical restaging (CT scan, examination under anesthesia and TURBT)
- Frequent follow-up
- Multiple invasive procedures
- That cystectomy might still be needed
- Uncertainty of tumor relapse
- Possible increased mortality
Presented by: Mitchell Benson, MD, Columbia University, USA
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre @GoldbergHanan at the 2018 FOIU 4th Friends of Israel Urological Symposium, July 3-5. 2018, Tel-Aviv, Israel
References:
1. Grossman HB et al. NEJM 2005
2. Herr H. Eur Urol 2008


