There are also plenty of trials assessing the role of ICI in 1st line therapy for MRCC (Table 1), demonstrating an added benefit for Ipilimumab/nivolumab in International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) intermediate and poor risk disease (Table 2). This clear benefit was shown in the Checkmate 214 trial comparing nivolumab/ipilimumab to sunitinib. This benefit was evident even when stratified according to the PD-L1 status (<1% and >=1%). The health-related quality of life for patients was also demonstrated to be better in the nivolumab/ipiliumb.
Table 1- Checkpoint inhibitor combination trials in 1st line MRCC:
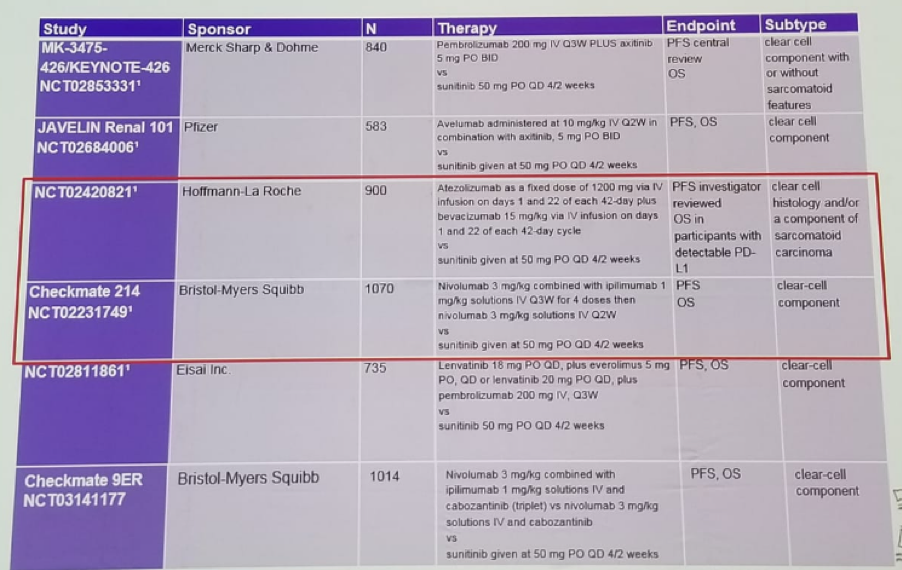
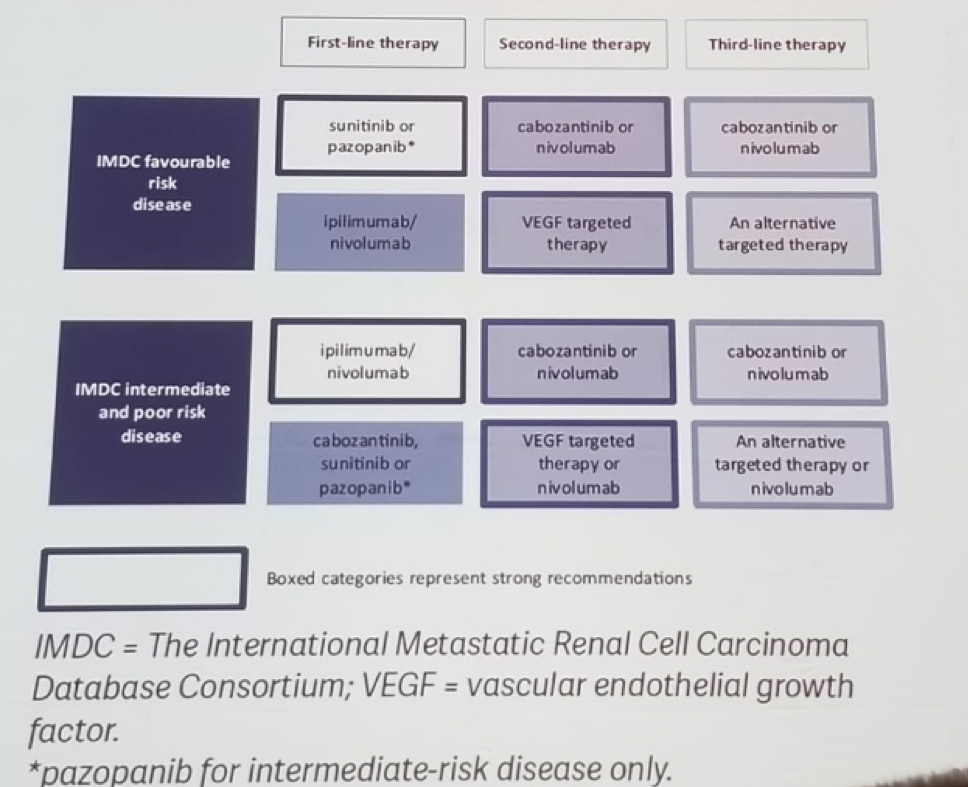
Table 2 – Updated EAU guidelines recommendations for the treatment of 1st line MRCC:
Another trial is the IMmotion 151 comparing atezolizumab +bevacizumab to sunitinib in clear-cell or sarcomatoid MRCC patients (figure 1).
Figure 1 – INMOTION 151 trial design:

The majority of patients in both Checkmate 214 and INMOTION 151 are either IMDC intermediate or poor-risk. In the INMOTION 151 trial, a clear advantage in progression-free survival (PFS) was seen for the atezolizumab + bevacizumab group (with a median PFS of 11.2 vs. 8.4 months). Again, health-related quality of life was better in the atezolizumab group.
It is not clear whether PD-1 blockade based combinations in MRCC are additive or synergistic. VEGF + PD-1 are certainly additive with encouraging objective response rate (ORR) and metastatic PFS (MPFS). However, it is not clear if these are synergistic. The overall survival (OS) benefit in Checkmate 214 is independent of PD-L1 tumor expression. INMOTION 151 lacks mature OS data and no significant PFS difference across all PD-L1 groups.
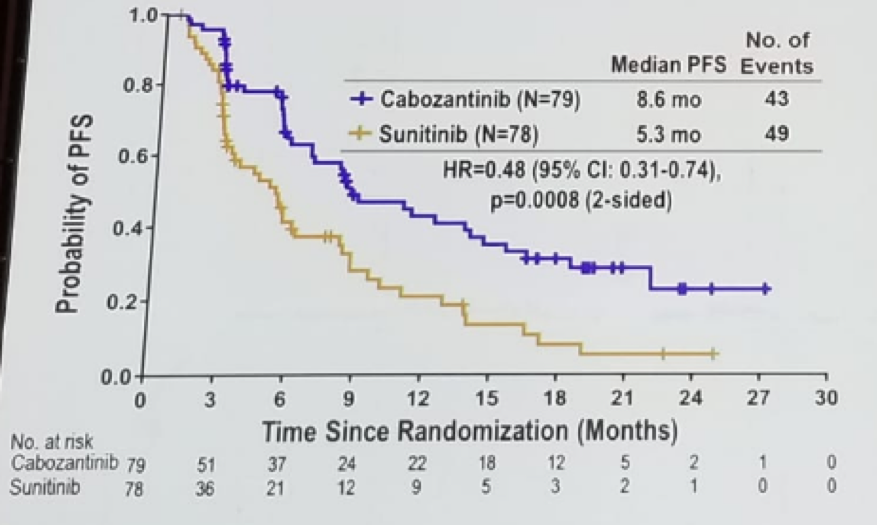
Bex continued to discuss the CABOSUN trial comparing cabozantinib vs. sunitinib in IMDC intermediate and poor risk patients. This demonstrated a clear advantage to cabozantinib in PFS (Figure 2), however no statistical significance in the OS difference was noted (hazard ratio of 0.8 (95% C.I. 053-1.21, p=0.29).
Figure 2: CABOSUN trial comparing cabozantinib to sunitinib in IMDC intermediate and poor risk MRCC patients:
There are molecular subtypes of clear cell RCC associated with sunitinib response in the metastatic setting. These entail ccrcc1/ccrcc4 tumors with lower relative risk, shorter PFS, and OS than ccrcc2/ccrcc/3, when treated with sunitinib. ccrcc4 is a strong inflammatory, TH1-oriented but suppressive immune microenvironment, with high expression of PD-1, and its ligands.
Bex summarized his great talk with a few points. Based on high-level evidence, if no drug-related contraindications are present, the IMDC prognostic model is currently the only tool for patient selection. ICI is effective irrespective of the expression of tumor or immune-cell PD-L1. Angiogenic or immune-inflammatory genotypes are emerging but have not been tested in phase 3. Lastly, subgroup analysis is interesting but cross-trial comparisons should not be done.
Presented by: Axel Bex, MD, The Netherlands Cancer Institute
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre @GoldbergHanan at the 2018 FOIU 4th Friends of Israel Urological Symposium, July 3-5. 2018, Tel-Aviv, Israel


