In the past, when IL-2 was used, CN was considered if tumor debulking could have resulted in more than 75% of the disease being removed, and patients had an ECOG of 0 or 1, and they had adequate organ function with no bony, liver, or CNS metastasis. 2 The two relevant randomized controlled trials by SWOG and EORTC were conducted in the IL-2 era. 3,4 The CARMENA trial seeks to help answer the question of whether or not CN should be performed prior to systemic therapy in the modern era.
CARMENA was a randomized phase III clinical trial which enrolled patients with synchronous clear cell MRCC. Inclusion criteria included an ECOG performance status of 0-1, absence of symptomatic brain metastases, acceptable organ function, and patients needed to be eligible for sunitinib therapy. Patients were randomized in a 1:1 fashion to sunitinib alone or CN followed by sunitinib. For those patients receiving surgery, sunitinib was to be initiated four to six weeks after surgery, at a 50 mg dose, on a 4 weeks on 2 weeks off schedule. The primary endpoint of this study was overall survival (Figure 1).
Figure 1 – Trial consort diagram:

According to Mejean, there are 3 types of MRCC patients; those with a minimal metastatic burden and excellent performance status, those with a high metastatic burden and poor performance status, and those with a moderate amount of metastatic disease and good performance status. Mejean notes that the CARMENA trial sought to answer the question of whether or not CN is beneficial in the group of patients with a moderate amount of metastatic disease and good performance status.
The trial took place between 2009 and 2017, and 450 patients had been enrolled. The median age of patients was 62. Most patients had an ECOG of 0 (56%). Based on the MSKCC risk groups, 56% were an intermediate risk and 44% poor risk in CN arm vs 59% and 42% in the non-CN arm. In the CN arm, 6.7% of patients never received surgery and 22.5% never received sunitinib. In the non-CN arm, 17% received nephrectomy later on and 4.9% never received sunitinib. At the time of this analysis, overall survival for patients receiving sunitinib without nephrectomy was NOT inferior compared with upfront CN. Of note, the percentage of patients who had clinical benefit, defined by disease control beyond 12 weeks was 47.9% in the sunitinib arm vs 36.6% in the CN arm (p=0.022) (Figure 2). Due to this, Mejean suggested that CN should no longer be considered standard of care in MRCC, at least when medical treatment is required.
The CARMENA trial represents the best evidence to date regarding nephrectomy in the metastatic setting.
Figure 2 – Comparison of survival in the trial arms:
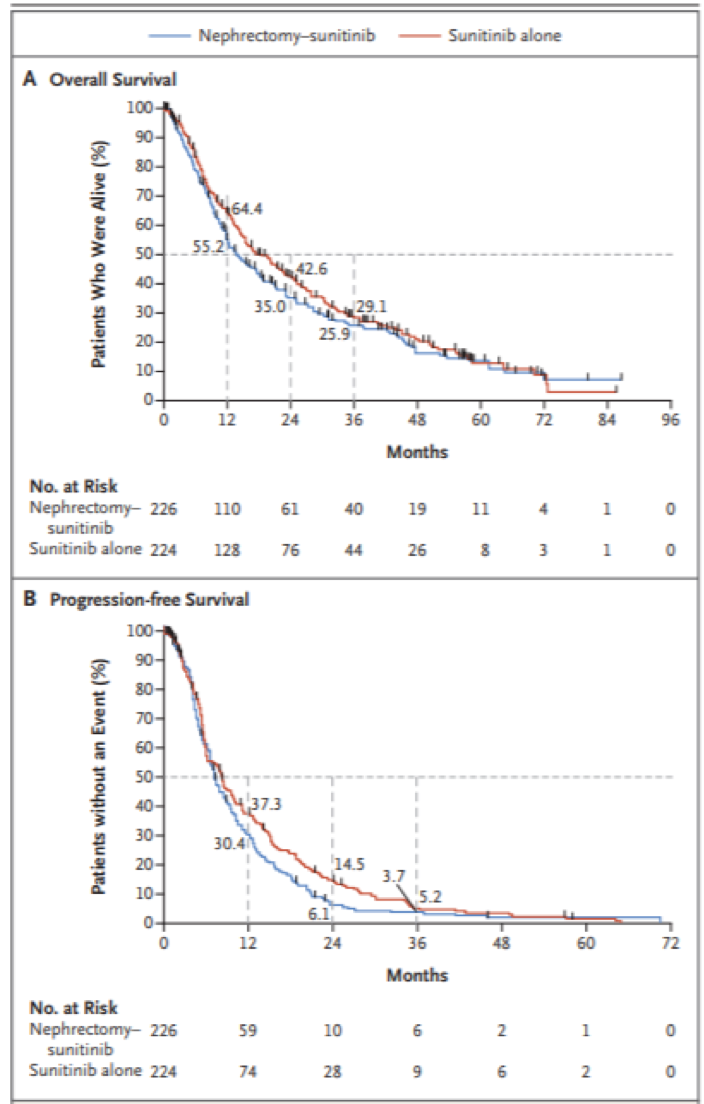
The major limitations of this study include the fact that since the beginning of this study, a number of other therapies have been introduced in the front-line setting of MRCC, including immunotherapy, which could significantly affect the prognosis and clinical pathway of these patients. Furthermore, the percentage of patients with poor- and intermediate-risk was very high in this study. No prospective data is available for the role of CN in patients with the limited metastatic disease.
References:
1. Choueiri TK, Xie W, Kollmannsberger C, et al. The impact of cytoreductive nephrectomy on survival of patients with metastatic renal cell carcinoma receiving vascular endothelial growth factor targeted therapy. The Journal of urology 2011;185:60-6.
2. Fallick ML, McDermott DF, LaRock D, Long JP, Atkins MB. Nephrectomy before interleukin-2 therapy for patients with metastatic renal cell carcinoma. The Journal of urology 1997;158:1691-5.
3. Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. New England Journal of Medicine 2001;345:1655-9.
4. Mickisch G, Garin A, Van Poppel H, de Prijck L, Sylvester R. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. The Lancet 2001;358:966-70.
Presented by: Arnaud Méjean, MD, Paris Descartes University, Paris, France
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre @GoldbergHanan at the 2018 FOIU 4th Friends of Israel Urological Symposium, July 3-5. 2018, Tel-Aviv, Israel


