- Local therapy only cures non-metastatic patients
- The timing of systematic therapy affects the prognosis
Overall survival (OS) benefit has been shown in many castration-resistant prostate cancer (CRPC) trials (Table 1). The Latitude trial has demonstrated an OS benefit for metastatic castrate sensitive PC patients with the usage of Abiraterone. 1
Table 1 – Overall survival benefit in CRPC trials:

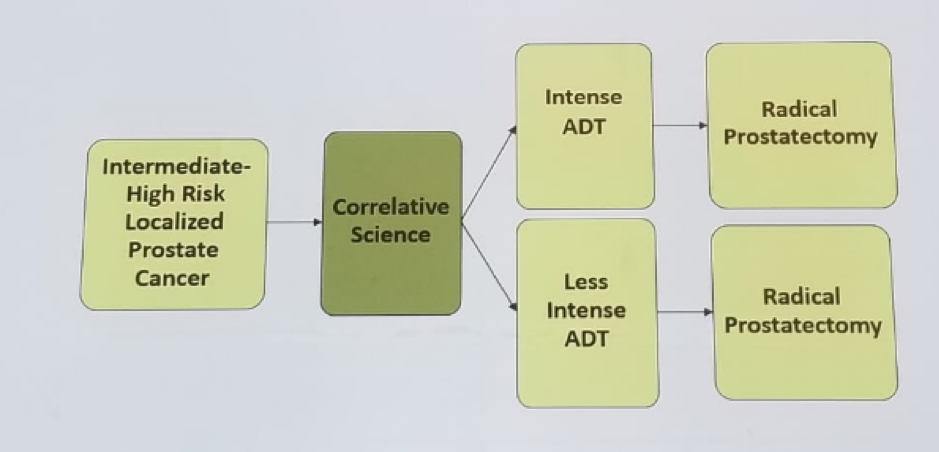
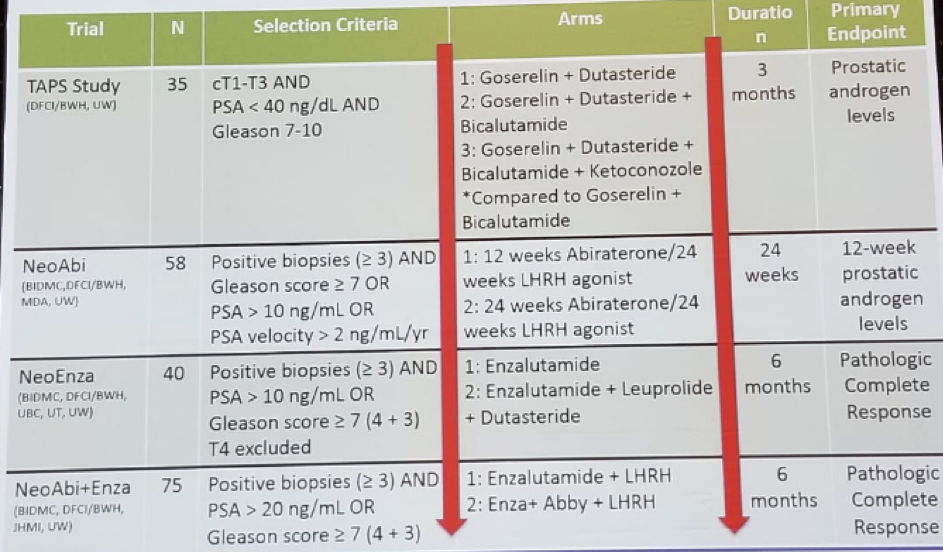
Kibel then described 4 different neoadjuvant trials, all sharing the same paradigm (Figure 1). The primary objective is to assess tissue androgen levels and assess pathological complete response and minimal residual disease rate. The targeted androgen pathway suppression (TAPS) study includes patients with clinically localized prostate cancer with clinical stage T1-T3 with Gleason >-7 and PSA < 40, randomized to LHRH agonist and:
- Bicalutamide
- Dutasteride
- Bicalutamide and Dutasteride
- Bicalutamide and Dutasteride and ketoconazole
Figure 1 – Neoadjuvant trial paradigm:
The maximal androgen blockade with abiraterone before RP is a similar study including men with clinically localized high-risk PC and at least 3 cores of Gleason 7 or above, or clinical stage T3 or PSA velocity of more than 2 ng/ml/year. Patients are then randomized to either ADT alone for 3 months or ADT +abiraterone. In the next step, these men undergo a biopsy and again receive ADT + abiraterone for 3 months, and then all men undergo prostatectomy. This study demonstrated that abiraterone treatment yielded significantly lower levels of all androgen when compared to LHRH alone. 3 The pathological complete response rate was 10% in the combined arm.
The third trial was a neoadjuvant complete androgen pathway suppression trial. This included patients with clinically localized high-risk PC with >3 cores of Gleason score 7 or above, or PSA above 10 ng/ml. Men were randomized to either enzalutamide for 6 months or LHRH agonist +Dutasteride + enzalutamide for 6 months. Then all men would undergo prostatectomy. The endpoints were tissue androgens and pathological complete response rate and minimal residual disease rates. Again, the arm with combined treatment with enzalutamide yielded significantly lower levels of all androgens when compared to enzalutamide alone. The complete response rate was 4% in the combined arm. The PSA and pathological outcomes of these 3 trials are shown in Figure 2.
Figure 2 – PSA and pathological outcomes:

The 4th and last neoadjuvant trial design are shown in Figure 3. The primary objective was to assess the pathological complete response rate and minimal residual disease rates between treatment arms. Overall 75 patients have been accrued so far. A summary of these 4 trials is shown in table 2.
The pathological complete response endpoint is an indicator of outcomes in several malignancies, including bladder cancer, esophageal, rectal, and breast. The FDA has approved pathological complete response as an endpoint for accelerated approval of new agents.
Figure 3 - Trial design in last neoadjuvant trial:

Table 2 – Phase 2 neoadjuvant trials:
Concluding this talk, Kibel said that the neoadjuvant trials demonstrate that longer and more intense ADT correlates with better pathological response. This, in turn, correlates with lower recurrence rates. Local therapy is effective, but early treatment of oligometastatic disease is the standard of care in many cancers, and it should be adopted in PC as well.
References:
1. Fizazi et al. NEJM 2017
2. Mostaghel EA. Et al. JCO 2014
3. Taplin ME et al. JCO 2014
Presented by: Adam Kibel, MD, Dana Farber Cancer Institute, USA
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre @GoldbergHanan at the 2018 FOIU 4th Friends of Israel Urological Symposium, July 3-5. 2018, Tel-Aviv, Israel


