Likely:
- Improved quality of life, with the recovery of testosterone (hot flashes, libido, erectile function, frailty and more)
- Reduced morbidity and mortality (metabolic syndrome, CV disease, diabetes, bone mineral density loss)
- Reduced drug costs
- Re-exposure of stem/progenitor cells to androgen may prolong the duration of androgen dependence and delay in castration resistance(demonstrated in murine models)
- Opportunity for integrated therapy with cell cycle directed interventions
Table 1: Sex steroids as tumor suppressors in prostate cancer:
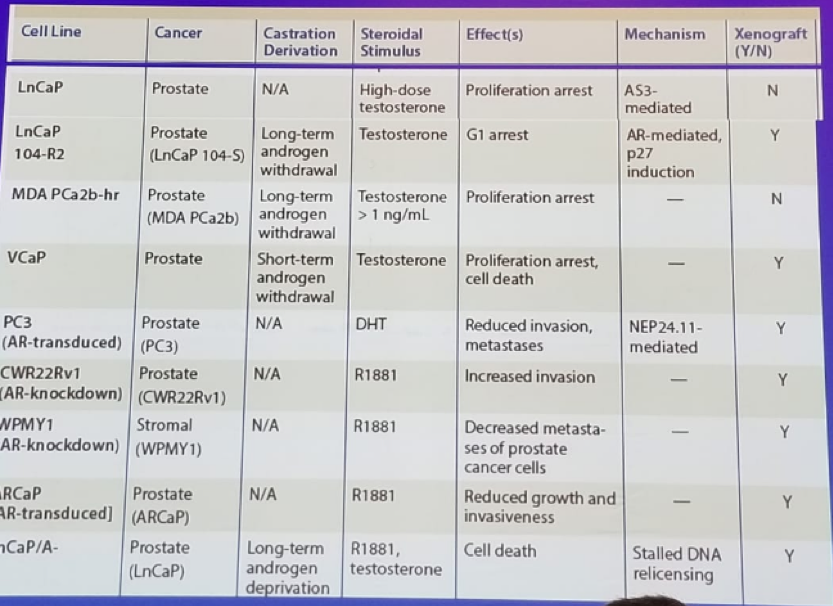
Table 2: Phase 3 trials of intermittent androgen deprivation therapy:
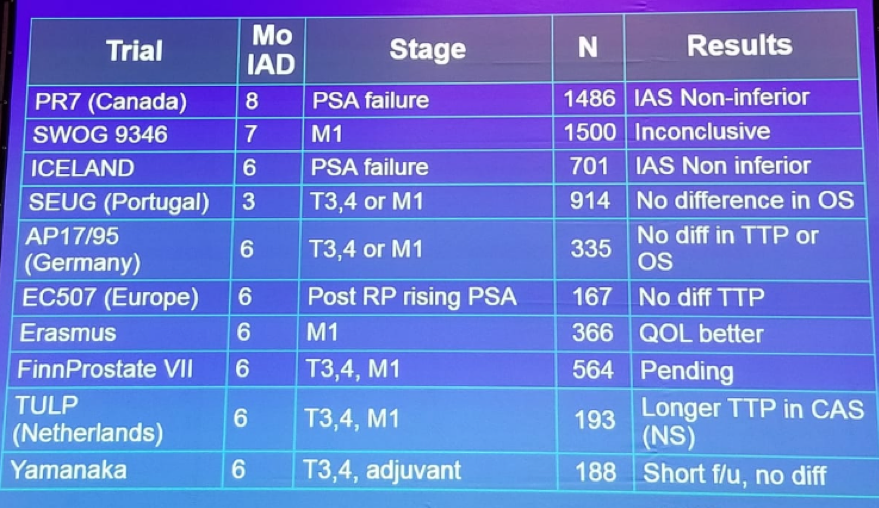
Figure 1: PR-7 - Overall survival in intermittent and continuous ADT:
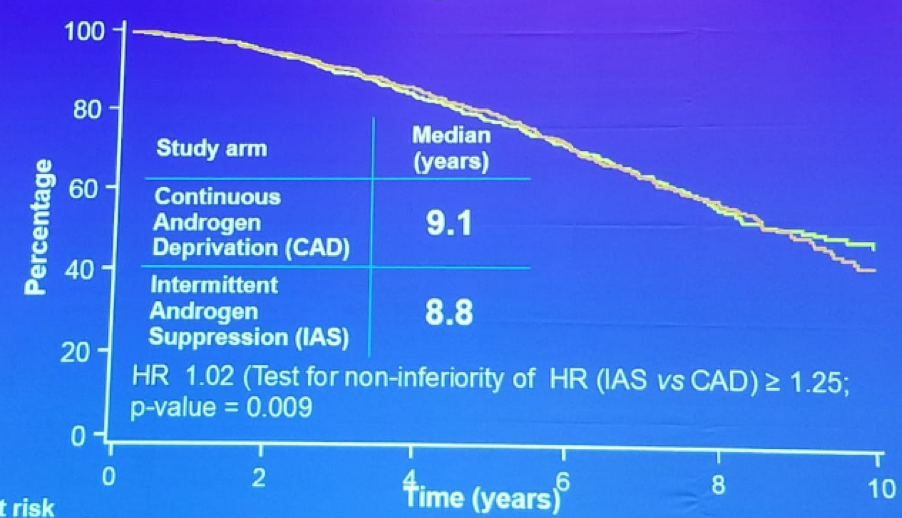
Figure 2: SWOG 9346 – Overall survival in intermittent and continuous ADT

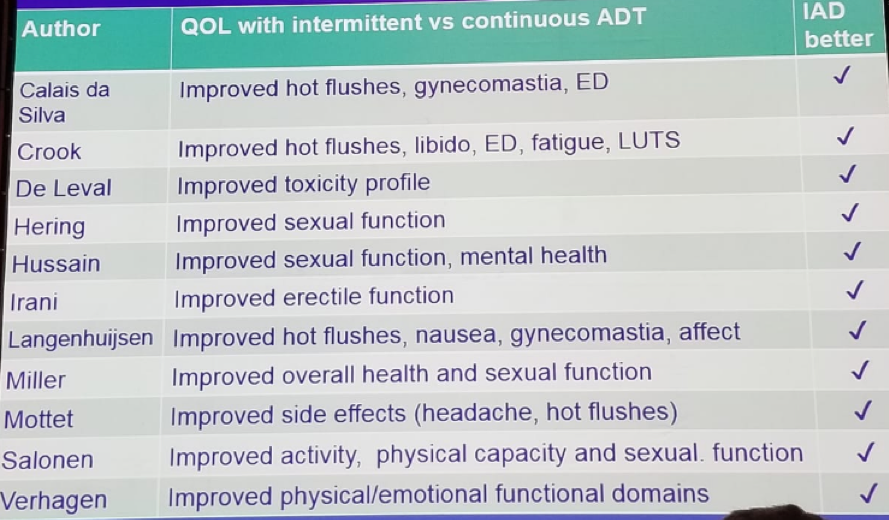
The logic behind the benefits of IADT includes the fact that testosterone has favorable health benefits, and there is extensive literature on the value of testosterone replacement. The improved quality of life associated with IADT when compared to continuous ADT has been shown in a plethora of trials (Table 3).
Table 3: Improved quality of life in intermittent ADT:
Klotz then discussed the recovery of testosterone in the off-treatment interval. Usually, testosterone does recover, as long as the induction period is modest (less than 1 year). Approximately 75-90% of patients recover to a normal range by 6 months. Recovery of serum testosterone to levels above 7.5 nmol/l (250 ng/dl) has been observed in 75%, 50%, 40%, and 30% of men in cycles 1-4, respectively. 3 According to Dr. Klotz, the recovery of testosterone is likely to have health benefits, which most likely include cardiovascular benefits as well. According to a population-based study comparing IADT to continuous ADT, there was a significant reduction in cardiovascular events with IADT compared to continuous ADT. 4 Additionally, there has been a published meta-analysis of a cardiovascular event in IADT vs. continuous ADT, demonstrating no difference in cardiovascular or thrombotic event rate, but reduced cardiovascular mortality with IADT. 5
Klotz concluded his great discussion reiterating that there is a clear quality of life and other health benefits in IADT, when compared to continuous ADT. IADT should be the standard treatment for non-metastatic disease and selected patients with metastatic disease. The most evidence clearly suggests that there are improved rates of cardiovascular events with IADT.
References:
1. Crook J, Klotz L. et al. NEJM 137 (10):2012
2. Hussain M. et al. NEJM 368;14 April 4, 2013
3. Bruchovsky N, Klotz L, et al. Cancer 2007
4. Tsai HT. et al. J Urol 2017
5. Jin C. et al. prostate Cancer Prostatic Disease 2016
Presented by: Laurence Klotz, MD, Sunnybrook Health Science Center, Toronto, Ontario, Canada
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre @GoldbergHanan at the 2018 FOIU 4th Friends of Israel Urological Symposium, July 3-5. 2018, Tel-Aviv, Israel


