- No flare phenomenon
- No issues in starting this therapy when baseline testosterone levels are over 5 ng/ml
- No issues in starting this therapy when baseline PSA levels are over 20 ng/ml
- Elevated alkaline phosphatase
Margel presented his own trial of early cardiovascular morbidity in a randomized trial comparing between LHRH-agonist and LHRH antagonist treatment in patients with advanced PC. The primary aim of this study was to compare endothelial function in patients with advanced PC and pre-existing CVD treated for one year with degarelix vs. LHRH agonist. The secondary endpoint was to compare CVE. This was a bi-central randomized open-label superiority study. Men with advanced PC and pre-existing CVD were included in this study. The men were stratified by cancer status (local vs. metastatic) and by endothelial function (measured by endoPAT). The inclusion and exclusion criteria are shown in Table 1. The outcomes included all cardiovascular events and all major adverse cardiovascular events (MACE) including:
- Death
- Myocardial infarction
- Cerebrovascular accident (CVA)
- Cardiac catheterization with stent
Table 1 – Inclusion and exclusion criteria:

Table 2 – Prostate cancer clinical details:
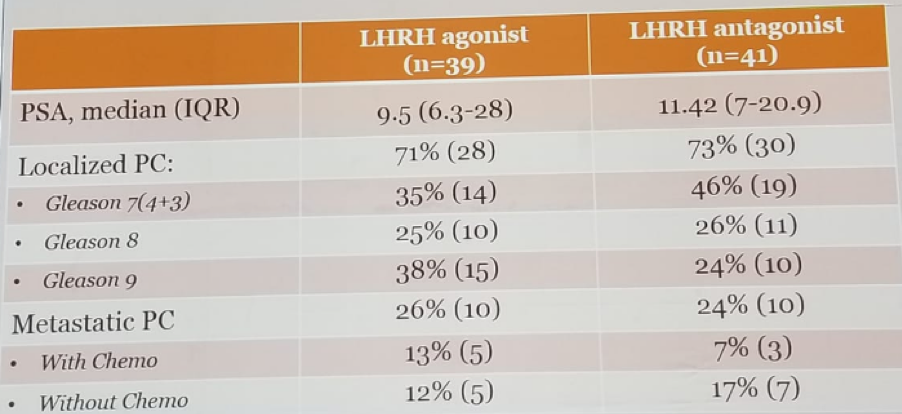
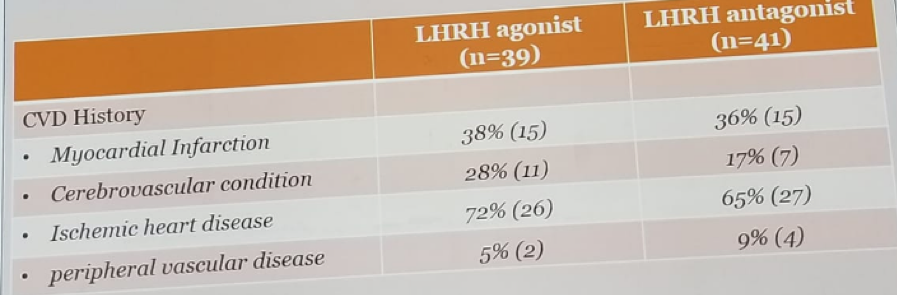
Table 3 – Cardiovascular disease details:
The results of the trial demonstrated that MACE had occurred in 20% of the agonist group compared to 2.4% of the antagonist group. A total of 35% CVE had occurred in the agonist group compared to 7% in the antagonist group. All differences were statistically significant. Interestingly NTproBNP, a well-known CVE predictor, was significantly higher in the agonist group (Figure 1).
Figure 1 – NTproBNP in the agonist and antagonist groups:
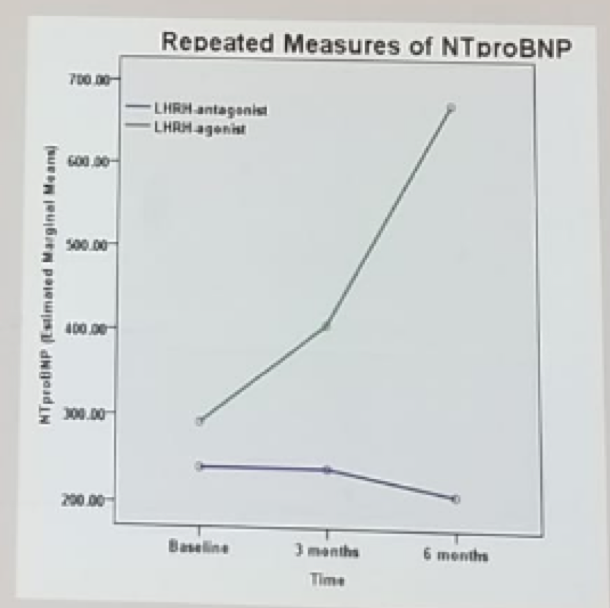
Margel suggested that the mechanism underlying these results are the GnRH receptor on the endothelium, macrophages and T-cells, and the fact that higher FSH levels, which are present with agonists, but not with antagonists, result in an increased metabolic risk.
Margel concluded his presentation, stating that most patients with PC die of heart disease. A plethora of evidence, including the presented prospective trial, suggest that antagonists are better than agonists, at least for those with previous ischemic heart disease.
Presented by: David Margel, MD, Rabin Medical Center, Israel
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre @GoldbergHanan at the 2018 FOIU 4th Friends of Israel Urological Symposium, July 3-5. 2018, Tel-Aviv, Israel
Read the Opposing Argument: Should Antagonists be the Primary Choice for ADT? - NO


