Data were abstracted from two double-blind, placebo-control studies of desmopressin. The first study enrolled females with 2 or more nocturic episodes per night over 3 days, who received 25mg of the medication or placebo. Male participants of the second trial were randomized to 50mg, 75mg or placebo. Researchers aimed to evaluate baseline parameters, adverse events, and an overall number of nighttime voids. The study population was defined as follows (Figure 1):
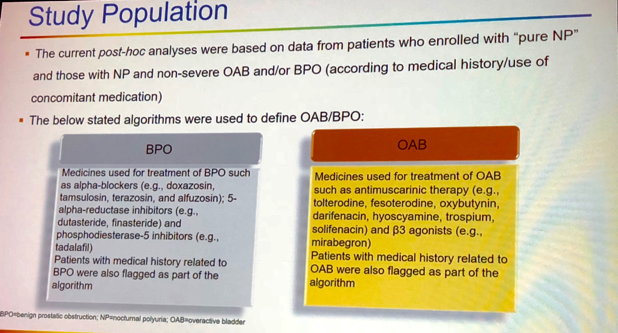
Figure 1
Two medication doses were analyzed for the purpose of this research: 25mg in women and 50mg in men. The study sample included 196 women with NP only, 35 women with NP and mild OAB, 152 men with NP only and 75 men with NP and mild OAB and/or BHO. Patients with severe OAB and BHO were excluded. Results demonstrate that desmopressin groups improved in terms of nocturnal episodes compared to the placebo arm (Figure 2).
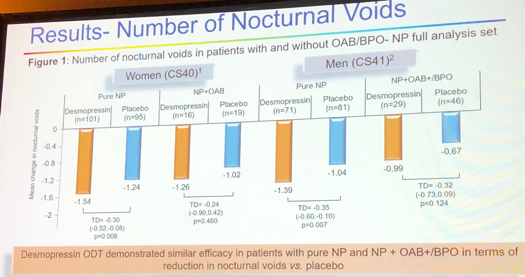
Figure 2
There was an increase in the proportion of patients achieving a 33% reduction of nocturic voids (Figure 3). The participants with NP and OAB/BHO reported no significant adverse events related to medication use.
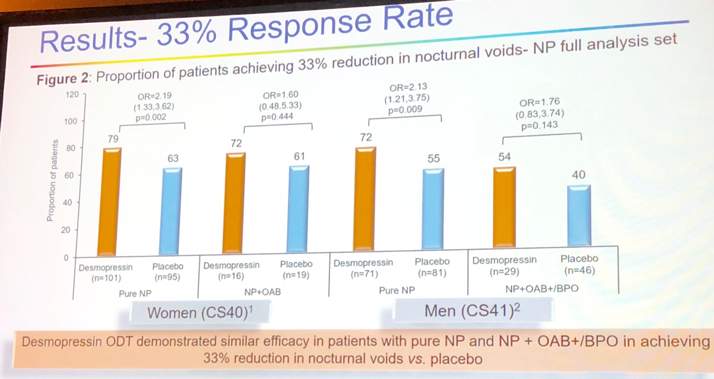
Figure 3
Future research is needed to evaluate the safety and efficacy of oral desmopressin in patients with more severe daytime urinary symptoms.
Presented by Jeffrey P. Weiss, MD, FACS, Department of Urology, SUNY Downstate Medical Center
Co-authors: Malmberg A, Juul K V, Ferring Pharmaceuticals, Denmark
Written by: Hanna Stambakio, BS, Clinical Research Coordinator, Division of Urology, University of Pennsylvania, @PennUrology at the 2018 ICS International Continence Society Meeting - August 28 - 31, 2018 – Philadelphia, PA USA


