(UroToday.com) In a podium presentation at the 2022 American Urologic Association Annual Meeting held in New Orleans and virtually, Dr. Chamie presented the results of N-803 in combination with BCG in patients with BCG-unresponsive non-muscle invasive bladder cancer (NMIBC).
This is a disease space in which there is a significant unmet need. Most patients newly diagnosed with bladder cancer have non-muscle invasive disease (NMIBC). For patients with intermediate or high-risk NMIBC and those with carcinoma in situ (CIS), adjuvant treatment with BCG is guideline-recommended based on proven benefits in disease recurrence. While BCG is efficacious, many patients eventually develop BCG-unresponsive disease and are at significant risk for recurrence, progression, and mortality. For many years, there have been very limited options for these patients. Radical cystectomy has remained the gold standard through numerous approaches including intravesical and systemic therapies (including pembrolizumab) have been investigated. N-803 (Anktiva) is a mutant IL-15-based immunostimulatory fusion protein complex (IL15RaFc) that promotes proliferation and activation of natural killer (NK) cells and CD8+ T cells, but not regulatory T cells.

In phase 1b trials of BCG-naïve patients with NMIBC, intravesical N-803 with BCG induced complete response in all patients that were durable for 24 months. Thus, this treatment approach was further explored in an open-label, 3 cohort multicenter study (QUILT 3.032) in patients with BCG-unresponsive high-grade NMIBC (NCT03022825).

In this presentation, the authors report data on 160 subjects who received intravesical N-803 plus BCG, consistent with the standard induction/maintenance treatment schedule. Patients were divided into two cohorts with differing primary outcomes: for patients with CIS (Cohort A) the primary outcome was incidence of complete response (CR) at any time while the primary endpoint for those with papillary disease (Cohort B) was the disease-free rate (DFS) at 12 months.
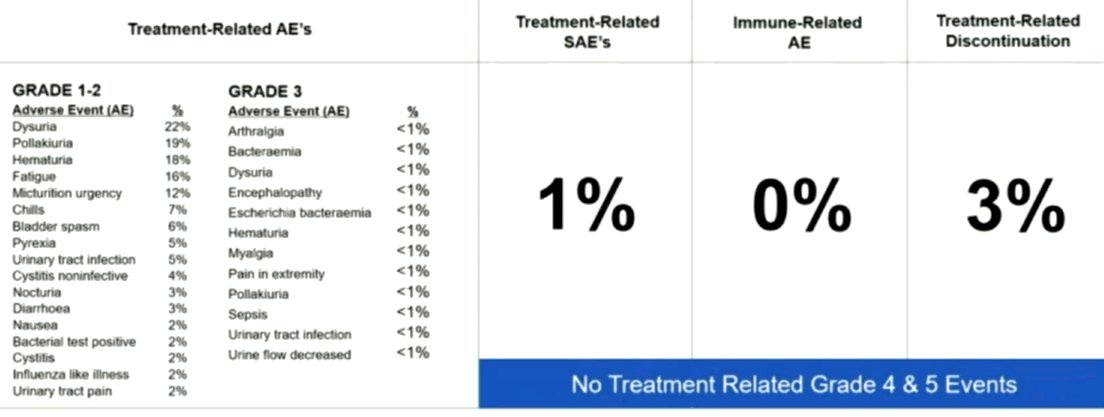
Of the 160 patients, 83 patients had CIS (Cohort A) and 77 had papillary disease (Cohort B). Together, the median age is 72.3 years and 81% are men. The mean number of prior TURBTs before enrollment was with a median of 12 prior BCG doses. Low grade treatment related AEs (grade 1-2) include dysuria (22%), pollakiuria (19%), hematuria (18%), fatigue (16%), and urgency (12%), all other AEs were seen at rates of 7% or less. No treatment related grade 4 or 5 AE was seen. No treatment emergent SAE's were considered treatment related. No immune related SAE's have been seen.
Among patients in Cohort A, patients with CIS had a CR rate of 71% (59/83). Among those that responded, the median duration of complete response was 24.1 months.

In this group, 91% avoided cystectomy, and 24 month bladder cancer specific progression free survival (defined as progression to MIBC) was 96%. The authors further demonstrated favourable outcomes for other survival endpoints.

The authors further demonstrated complete remission rates in this cohort were consistent across subgroups defined based on numerous stratification criteria.
In cohort B, patients with papillary disease had disease-free survival of 57% at 12 months and 48% at 24 months.
In this group, 95% avoided cystectomy during the observation period. Even among those undergoing cystectomy, this was delayed among responders (N=4) with a median time to surgery of 12.9 months versus 7.8 in non-responders (N=8).
Pharmacokinetic data show no systemic levels of N-803, confirming that this intravesical therapy is not absorbed.
Dr. Chamie concluded that, among 160 patients with BCG-unresponsive NMIBC, there is a 99% bladder cancer specific overall survival at 2 years after treatment with intravesical N-803 plus BCG. In patients with CIS, a 71% CR rate was seen with 24.1 months median duration of response and 96% absence of progression to MIBC at 24 months. A 53% DFS rate at 18 months was seen in those with papillary disease. Together, these results show that the efficacy and safety profile of N-803+BCG exceeds other available intravesical and systemic options.
Presented by: Karim Chamie, MD, University of California Los Angeles, Los Angeles, CAWritten by: Christopher J.D. Wallis, University of Toronto Twitter: @WallisCJD during the 2022 American Urological Association (AUA) Annual Meeting, New Orleans, LA, Fri, May 13 – Mon, May 16, 2022.


