(UroToday.com) The 2022 American Urological Association (AUA) Annual Meeting included a session on non-invasive bladder cancer and a presentation by Dr. Ian McElree discussing sequential intravesical gemcitabine + docetaxel for BCG-naïve high-risk non-muscle invasive bladder cancer (NMIBC). BCG is currently recommended as adjuvant therapy following complete transurethral resection of bladder tumor (TURBT) for high-risk NMIBC. However, production shortages have precluded BCG in many urologic practices, thus leading to urologists using gemcitabine + docetaxel in the BCG-naïve setting. At the AUA meeting, Dr. McElree report the outcomes of patients with high-risk BCG-naïve NMIBC treated with gemcitabine + docetaxel.
This study retrospectively reviewed all patients with BCG-naïve high-risk NMIBC treated with gemcitabine + docetaxel from May 2013 through April 2021. Patients received 6 weekly intravesical instillations of sequential 1 gram gemcitabine and 37.5 mg docetaxel after complete TURBT. Monthly maintenance of 2 years was initiated if disease free at first follow-up. The primary outcome was recurrence-free survival (RFS), and recurrence was defined as tumor relapse in the bladder or prostatic urethra. Progression was defined as T-stage increase or development of muscle invasive or metastatic disease. Survival was assessed with the Kaplan-Meier method, indexed from the first gemcitabine + docetaxel instillation.
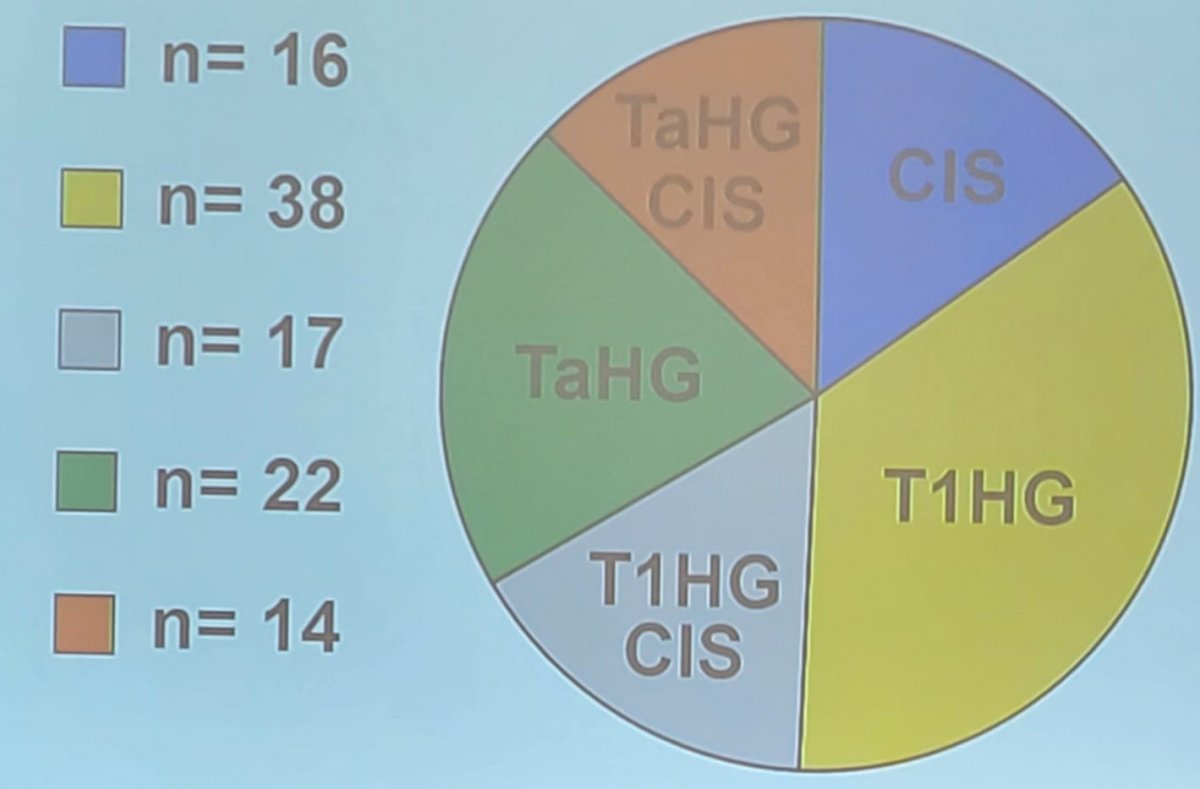
There were 107 patients included in the study, with a median age of 76 years, 94% receiving full induction, and included 8 patients with micropapillary features. Four patients did not complete a full induction cycle due to hematuria (n = 3) and severe frequency/nocturia (n = 1). The breakdown of the patients by pathology status is as follows:
After a median follow-up of 15 months (IQR 8-26 months), the RFS was 89%, 85%, and 82% at 6, 12, and 24 months, respectively:
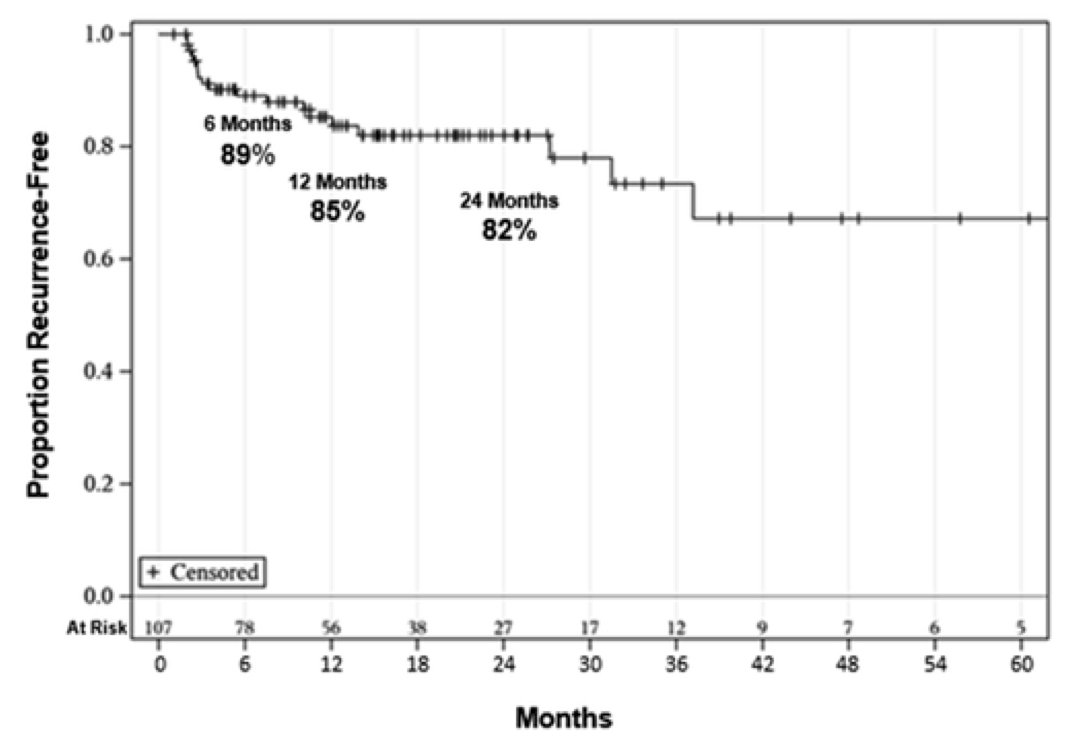
There was no difference in RFS seen in patients with or without CIS (p=0.42), and no patients had disease progression. One patient underwent cystectomy due to end-stage lower urinary tract symptoms (final pathology pTisN0), and no patients died of bladder cancer. The median duration of response was 91% at 2 years, and overall survival at 2 years was 84%. Among the 8 patients with micropapillary disease, 6 responded completely to gemcitabine + docetaxel and the two that immediately relapsed responded to valrubicin + docetaxel. 46 patients reported any symptoms during treatment, commonly urinary frequency/urgency (36%), hematuria (11%), and dysuria (8%).
Dr. McElree concluded his presentation discussing sequential intravesical gemcitabine + docetaxel for BCG-naïve high-risk NMIBC with the following take-home messages:
- In a large cohort of high-risk, BCG-naïve NMIBC patients, gemcitabine + docetaxel showed excellent efficacy and durability (82% 2-year RFS)
- Prospective comparative analysis of gemcitabine + docetaxel in BCG-naïve populations is warranted
Presented By: Ian M. McElree, BS, MS, University of Iowa, Iowa City, IA
Co-Authors: Ryan L. Steinberg, Alexander C. Martin, Jordan Richards, Sarah L. Mott, Paul T. Gellhaus, Kenneth G. Nepple, Michael A. O'Donnell, Vignesh T. Packiam, Iowa City, IA
Written By: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Urological Association (AUA) Annual Meeting, New Orleans, LA, Fri, May 13 – Mon, May 16, 2022.


