(UroToday.com) The 2022 American Urological Association (AUA) Annual Meeting included a session on advanced kidney cancer and a presentation by Dr. Kazuyuki Numakura discussing immune-related adverse events and survival outcomes for metastatic renal cell carcinoma (RCC) patients treated with nivolumab plus ipilimumab. A combination immune therapy with nivolumab + ipilimumab is first-line treatment for metastatic RCC patients with IMDC intermediate and poor risk. Almost 40% of patients achieve a durable response, and median overall survival expects nearly four years. On the other hand, since 20% of patients have resistance to nivolumab + ipilimumab, a predictive marker for clinical outcomes of nivolumab + ipilimumab is necessary. Furthermore, the impact of immune-related adverse events occurring from nivolumab + ipilimumab has not been thoroughly investigated. Thus, Dr. Numajura and colleagues evaluated the clinical implication of immune-related adverse events in metastatic RCC patients to better select candidates for nivolumab + ipilimumab.
This study retrospectively evaluated 89 patients with metastatic RCC treated with nivolumab + ipilimumab from multiple institutions. The associations between immune-related adverse events and progression-free survival (PFS), overall survival (OS), and objective response rates (ORRs) were analyzed.
The median follow-up period was 15.1 months (range: 1 to 74 months), with a total of 82 immune-related adverse events occurring in 56 patients (63%). PFS and OS were significantly longer in patients with immune-related adverse events than that in patients without immune-related adverse events (HR 0.43, 95% CI 0.22-0.82 and HR 0.42, 95% CI 0.19-0.93) respectively:
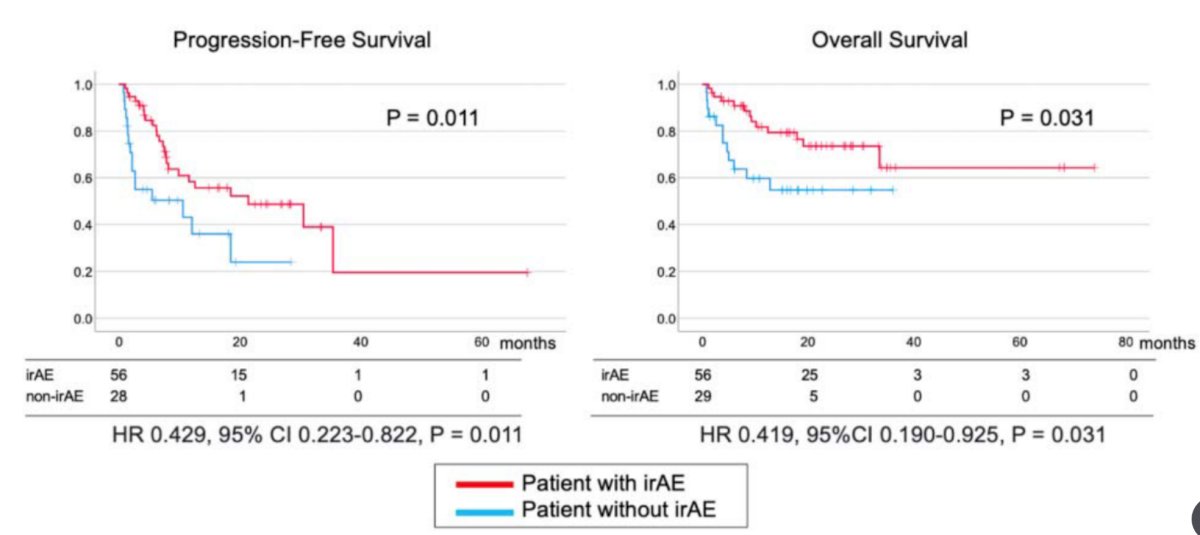
On univariate analysis, lymph node metastasis (HR 3.05, 95% CI 1.36-6.91), high CRP (HR 1.59, 95% CI 1.06-2.37), prior nephrectomy (HR 2.76, 95% CI 1.09-6.99), immune-related adverse events (HR 0.42, 95% CI 0.19-0.93), and variant histology (HR 3.06, 95% CI 1.15-8.19) were associated with OS. On multivariable analysis, the development of immune-related adverse events was an independent predictor of a longer OS (HR 0.40, 95% CI 0.17-0.93). Additionally, ORR was better in patients with immune-related adverse events (OR 3.94, 95% CI 1.38-11.19).
Dr. Numakura concluded this presentation by assessing immune-related adverse events and survival outcomes for metastatic RCC patients treated with nivolumab plus ipilimumab with the following take-home messages:
- This retrospective study showed that immune-related adverse events were independently associated with PFS and OS in patients treated with nivolumab + ipilimumab as first-line therapy
- The development of immune-related adverse events may portend better survival outcomes
Presented by: Kazuyuki Numakura, MD, Department of Urology, Akita University Graduate School of Medicine, Akita, Japan
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Urological Association (AUA) Annual Meeting, New Orleans, LA, Fri, May 13 – Mon, May 16, 2022.


