(UroToday.com) The 2022 American Urological Association (AUA) Annual Meeting included a session on advanced prostate cancer and a presentation by Dr. Neal Shore discussing oral relugolix for ADT in advanced prostate, specifically a pooled safety analysis from randomized trials.
Relugolix is a highly selective, orally active, nonpeptide GnRH receptor antagonist approved for the treatment of men with advanced prostate cancer. In the phase 3 HERO study, relugolix demonstrated sustained testosterone suppression superior to that of leuprolide, a safety profile consistent with its mechanism of action, and a 54% decrease in risk of major adverse cardiovascular events relative to leuprolide.1 In order to provide a detailed summary of the relugolix safety profile, Dr. Shore and colleagues analyzed pooled data from 2 randomized relugolix clinical trials.
The pooled safety analyses include data from 1,012 men with advanced prostate cancer: 934 men (relugolix: 622; leuprolide: 308) from the phase 3 HERO study and 78 men (relugolix: 54; leuprolide: 24) from the C27002 randomized phase 2 study. As follows are the two trial schema for C27002 and HERO:
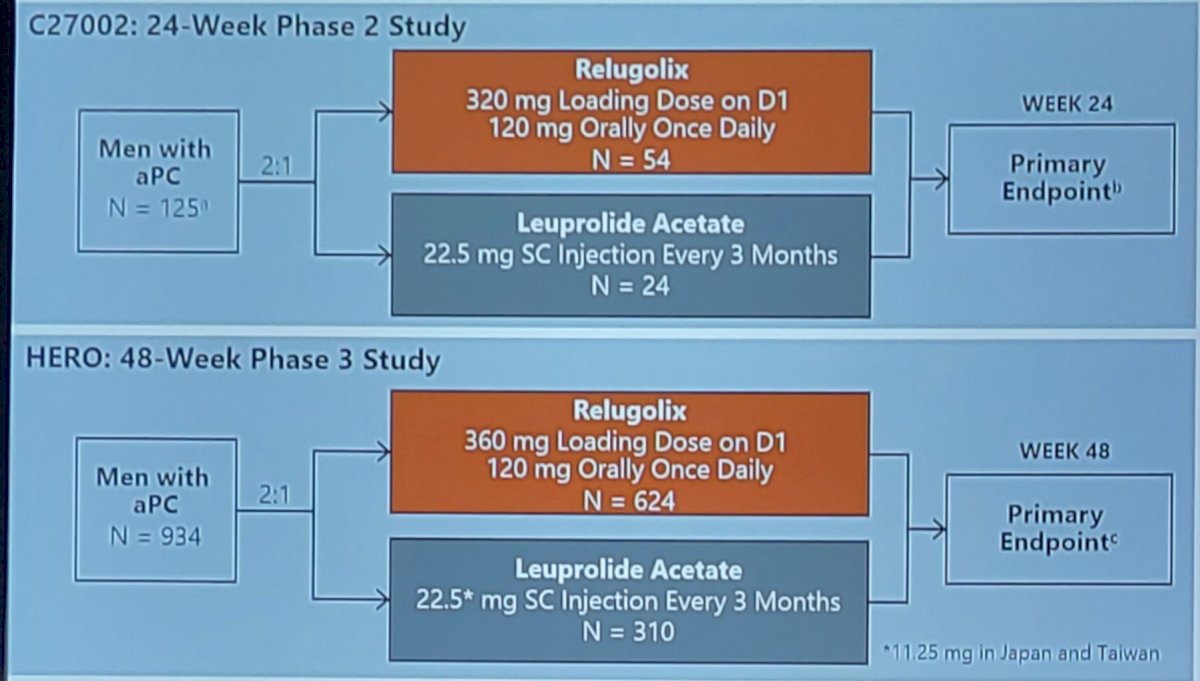
Only men who received either relugolix 120 mg orally once daily (360 mg day 1 loading dose) or leuprolide injections every 12 weeks for 24-48 weeks were included in the analyses. Safety assessments included clinical laboratory tests, reporting of adverse events, and major adverse cardiovascular events (defined as nonfatal myocardial infarction, non-fatal stroke, and death from any cause). Individual patient data were pooled and results of all analyses were summarized descriptively.
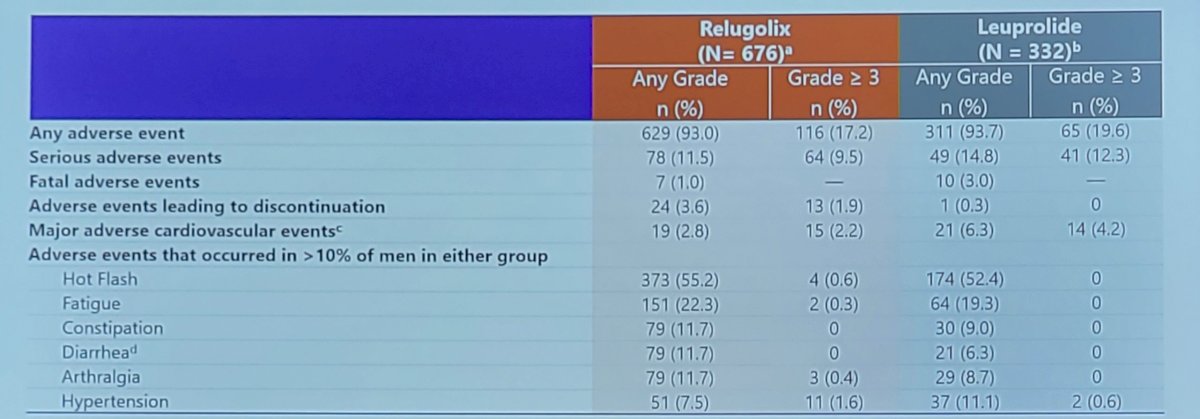
The overall incidence of adverse events was 93.0% and 93.7% in the relugolix and leuprolide groups, respectively. Hot flash was the most common adverse events in both groups (55.2% in the relugolix group and 52.4% in the leuprolide group). Grade ≥ 3 adverse events occurred in 17.2% and 19.6% in the relugolix and leuprolide groups, respectively. The most common grade ≥ 3 adverse events were hypertension (1.6% vs 0.6%) and syncope (1.0% vs 0.9%). Fatal events were reported for 1.0% of men in the relugolix group and 3.0% in the leuprolide group. Major adverse cardiovascular events incidence in this pooled analysis was 2.8% in the relugolix group and 6.3% in the leuprolide group. As follows is a table summarizing this safety data:
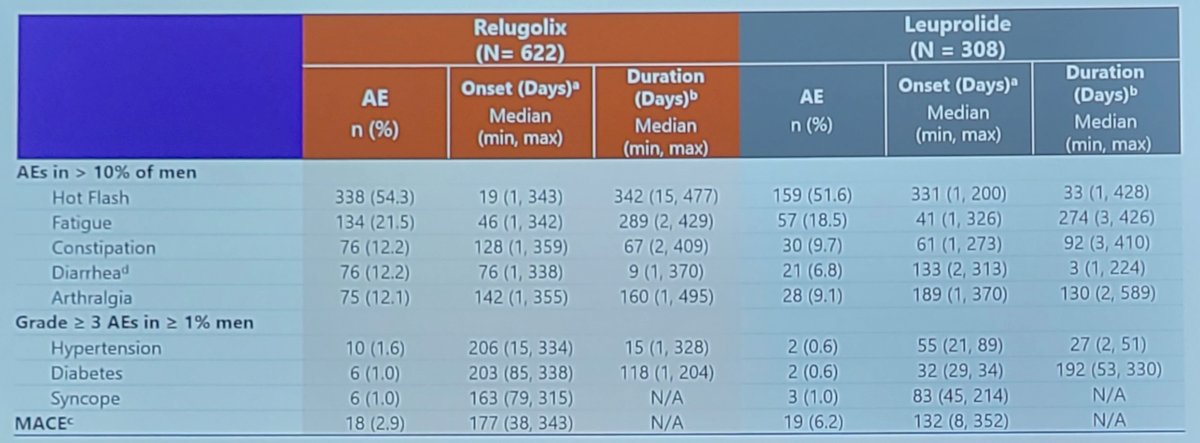
As follows are the results for specific adverse events of interest, with notably diarrhea favoring leuprolide and major adverse cardiovascular events favoring relugolix:

With regards to onset and duration of adverse events, Dr. Shore highlighted data from the HERO study, specifically a longer onset to major adverse cardiovascular event:
Dr. Shore concluded his presentation by discussing safety data from pooled trial data for relugolix with the following take-home messages:
- Relugolix is an FDA approved, first-in-class oral, highly selective gonadotropin-releasing hormone antagonist that is given once daily
- In the pooled analyses of 2 randomized clinical studies in advanced prostate cancer, relugolix was generally well tolerated, with a lesser incidence of major adverse cardiovascular events relative to leuprolide
Presented By: Neal D. Shore, MD, FACS, Carolina Urologic Research Center, Myrtle Beach, SC
Co-Authors: James L. Bailen, Jeffersonville, IN; Fred Saad, Montreal, Canada; Daniel J. George, Durham, NC; Bryan Mehlhaff, Springfield, OR; Michael S. Cookson, Oklahoma City, OK; Daniel R. Saltzstein, SanAntonio, TX; Ronald Tutrone, Towson, MD; Bruce Brown, Andria G. M. Langenberg, Sophia Lu, Jina Lee, Brisbane, CA; Sarah Hanson, New York, NY; Bertrand Tombal, Brussels, Belgium
Written By: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Urological Association (AUA) Annual Meeting, New Orleans, LA, Fri, May 13 – Mon, May 16, 2022.


