(UroToday.com) The 2024 South Central AUA annual meeting included a session on prostate cancer, featuring a presentation by Dr. Elizabeth Wulff-Burchfield discussing clinical trial updates for treatment of metastatic prostate cancer. Dr. Wulff-Burchfield started her presentation by highlighted that she would be discussing recent, high impact systemic therapy trials for three different disease states:
- High risk biochemical recurrence
- Metastatic, hormone sensitive prostate cancer (mHSPC)
- Metastatic castrate-resistant prostate cancer (mCRPC)
In the high risk biochemical recurrence/non-metastatic HSPC disease space, EMBARK1 was a phase 3 trial that enrolled patients with a PSA ≥1 ng/ml after radical prostatectomy or ≥2 ng/ml above nadir after primary external beam radiotherapy, with a PSA doubling time of ≤9 months. Patients had no evidence of metastasis on conventional imaging and baseline testosterone was ≥150 ng/dL. Hormone therapy ≥9 months prior to enrolment was permitted. Patients underwent stratified randomization (by PSA level, PSA doubling time, and prior hormonal therapy receipt) to one of three arms:
- Enzalutamide 160 mg (standard dose) + leuprolide (blinded arm)
- Placebo + leuprolide (blinded)
- Enzalutamide monotherapy (unblinded)
PSA was assessed at 36 weeks, and if patients had a PSA < 0.2 ng/mL treatment was suspended at week 37 and PSA was monitored with treatment reinitiated if PSA increased again. If patients had a PSA > 0.2 ng/mL treatment was continued. The primary endpoint was metastasis-free survival, assessed via blinded independent central review, in the enzalutamide + leuprolide versus leuprolide arms only. Key secondary endpoints included overall survival and safety outcomes. The trial design for EMBARK is as follows:

At a median follow-up of 5 years, the combination of enzalutamide + leuprolide versus leuprolide alone demonstrated a significant improvement in metastasis free survival (HR 0.42, 95% CI 0.31 – 0.61, p < 0.0001). The median metastasis free survival was not reached in either arm to date:

Comparisons between the enzalutamide monotherapy and leuprolide monotherapy arms were also performed. This demonstrated prolonged metastasis free survival in the enzalutamide only arm, with a HR of 0.63 (95% CI 0.46 – 0.87, p = 0.0049):

Among the secondary endpoints, generally these were improved with enzalutamide + leuprolide or enzalutamide alone versus leuprolide alone. In the enzalutamide + leuprolide group, 90.9% of patients had treatment suspended for a median of 20.2 months (range: 5.7 to 87.9), compared to 85.9% in the enzalutamide monotherapy group (for a median of 11.1 months, range: 2.3 to 84.9). At ESMO 2024, Dr. Neal Shore presented additional EMBARK data, noting that serious adverse events were more common in patients aged ≥70 years vs <70 years for enzalutamide + leuprolide (45.0% vs 25.3%), leuprolide alone (36.2% vs 27.1%), and enzalutamide monotherapy (43.9% vs 30.4%).
During treatment suspension in the combination group and the leuprolide-alone group, testosterone recovered slightly, but not to baseline levels. There was very little effect on testosterone in the enzalutamide monotherapy group. Once treatment resumed in both groups (enzalutamide + leuprolide and leuprolide alone), testosterone levels were reduced:

Dr. Wulff-Burchfield then discussed the PATCH trial, which was presented at ESMO 2024. PATCH was an open-label, randomized phase 3, non-inferiority comparison of LHRH agonist versus transdermal estradiol patches:

Eligibility criteria included histologically confirmed newly diagnosed high-risk M0 (locally advanced or node positive) prostate cancer or those relapsing with PSA ≥ 4 ng/ml and doubling in <6 months, PSA ≥ 20 ng/ml, or N positive. Treatment included standard LHRH agonist versus transdermal estradiol 100 mcg/24 hour patches (four patches) changed twice weekly for ≥2 years (when testosterone <= 1.7 nmol/L, 3 patches were used and changed twice weekly); prostate radiotherapy and docetaxel were permitted. The primary outcome was metastasis-free survival, designed to rule out a >4% reduction in 3-year metastasis-free survival (85% power, 1-sided 5% α). Secondary outcomes included overall survival, castration rates, and toxicity.
There were 1,360 men, [639 LHRH agonist, 721 transdermal estradiol (randomization ratio 1:2 then 1:1)] recruited from PATCH (NCT00303784, n = 1,082) and STAMPEDE (NCT00268476, n = 278) trial sites between 2007-2022. The LHRH agonist 3-year metastasis-free survival rate was 87% (giving a target non-inferiority HR of 1.31). Transdermal estradiol 3-year metastasis-free survival was 86% HR 0.96 (95% CI 0.81-1.14) in favor of transdermal estradiol, excluding a 2% reduction in metastasis-free survival:

Overall survival was HR 0.89 (95% CI 0.74-1.07) in favor of transdermal estradiol:

Sustained castration rate was defined as testosterone ≤1.7 nmol/L over 1 year; n = 1,066), with transdermal estradiol use confirmed as estradiol ≥250 pmol/L; 85% for both groups. LHRH agonist versus transdermal estradiol any grade adverse events included gynecomastia 42% versus 85% and hot flushes 89% versus 44%, respectively. Transdermal estradiol improved bone mineral density, there was no excess cardiovascular toxicity, and there was improved overall quality of life scores. Specifically, the mean difference in 6-month overall score was +4.2 (1.2, 7.1) in favor of transdermal estradiol (p = 0.006).
Of note, there are several upcoming trials in the biochemical recurrent disease space to be aware of:
- NRG-GU011/NRG PROMETHEAN (NCT05053151): patients with biochemical recurrence with PET-only oligometastases randomized to stereotactic body radiotherapy + ADT (relugolix) for six months versus stereotactic body radiotherapy + placebo
- PSMA-DC (NCT05939414): patients with biochemical recurrence with PET-only oligometastases randomized to stereotactic body radiotherapy to all sites of PET-avid disease versus stereotactic body radiotherapy + 177Lu-PSMA-617
In the mHSPC disease space, Dr. Wulff-Burchfield discussed the ARANOTE trial, also presented at ESMO 2024 [2]. Eligible patients in ARANOTE had mHSPC by conventional imaging, an ECOG performance status of 0–2, and started ADT ≤ 12 weeks. Patients were randomized 2:1 to darolutamide 600 mg twice daily or placebo, each with ADT. The primary endpoint was radiological progression-free survival, and secondary endpoints included overall survival, time to initiation of subsequent anticancer therapy, time to CRPC, time to PSA progression, time to pain progression, and safety. The trial design for ARANOTE is below:
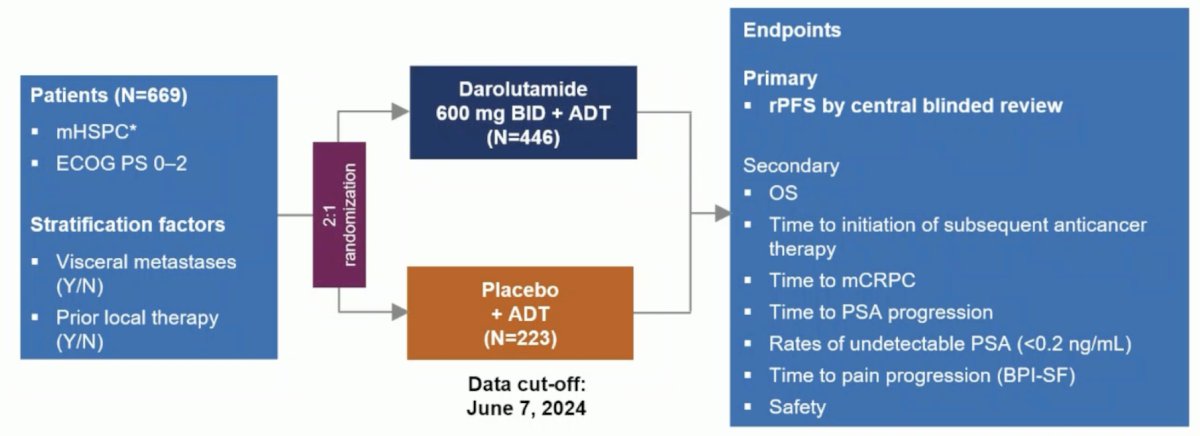
At the primary data cutoff of June 7, 2024, darolutamide + ADT significantly improved radiological progression-free survival versus placebo + ADT (HR 0.54, 95% CI 0.41–0.71):

Darolutamide was associated with a positive trend for overall survival (HR 0.81, 95% CI 0.59–1.12) and clinical benefits across all secondary efficacy endpoints, including time to CRPC (HR 0.40, 95% CI 0.32–0.51) and time to pain progression (HR 0.72, 95% CI 0.54–0.96). Additionally, there was a benefit favoring darolutamide + ADT for time to subsequent therapy (HR 0.40, 95% CI 0.29–0.56) and time to PSA progression (HR 0.31, 95% CI 0.23–0.41). Further, PSA < 0.2 ng/mL at any time during treatment occurred in 62.6% of darolutamide + ADT patients compared to 18.5% for placebo + ADT patients. Incidences of treatment-emergent adverse events were low and similar between groups, and treatment discontinuations due to treatment-emergent adverse events were lower in patients receiving darolutamide versus placebo (6.1% vs 9.0%). Treatment emergent adverse events associated with ARPIs were generally similar between treatment groups; fatigue was less common in the darolutamide + ADT group versus placebo + ADT.
Results of the UpFrontPSMA trial were also presented at ESMO 2024 [3]. This trial included patients with de novo high-volume mHSPC who had received ≤4 weeks of ADT and had a PSA >10 ng/ml at diagnosis. Prior to randomization patients underwent both PSMA and FDG PET scans. Eligibility was limited to those patients with evidence of high tumor uptake and high-volume disease on PET scans. Patients were also required to have the majority of their metastatic disease demonstrating PSMA positivity. Eligible patients were randomized to:
- Experimental arm: 177Lu-PSMA-617 7.5 GBq x 2 cycles + docetaxel 75 mg/m2 x 6 cycles
- Control arm: Docetaxel 75 mg/m2 x 6 cycles

Between May 2020 and April 2023, 130 patients were recruited and underwent randomization (experimental: 63, control: 67). Overall, 22% of screened patients failed on PET. The median follow-up was 2.5 years. All 63 patients in the experimental arm completed the two cycles of 177Lu-PSMA. 79% of patients in the combination arm completed all 6 cycles of docetaxel, compared to 84% of patients in the control arm. However, a docetaxel dose reduction was required in 33% of patients in the combination arm, compared to 17% of patients in the docetaxel arm.
For the primary outcome, an undetectable PSA at week 48 was observed in 41% of patients in the 177Lu-PSMA + docetaxel arm versus 16% of patients in the docetaxel control arm (OR 3.88, 95% CI 1.61–9.38, p = 0.002). An undetectable PSA at any point was observed in 51% and 32% of patients, respectively (OR 2.14, p = 0.042). There were no differences in undetectable PSA levels at week 12 between the two arms (p = 0.9).
Time-to-event analyses demonstrated that patients in the experimental arm had superior PSA progression-free survival (median: 31 versus 20 months; HR 0.60, p = 0.039) and freedom from castration resistance (HR 0.60, p = 0.033):


Radiographic progression-free survival similarly favored 177Lu-PSMA + docetaxel (HR 0.58, p = 0.067):

To date, there is no overall survival benefit with the addition of 177Lu-PSMA to docetaxel (HR 0.83, 95% CI: 0.38–1.83, p = 0.646):

Adverse events were similar in both arms, with grade 3–4 events in 27–29% of cases, and there were no new safety signals. As expected, patients in the 177Lu-PSMA arm had a higher frequency of dry mouth (37% versus 0% – all grade 1–2).
There are several ongoing key trials in the mHSPC disease space, highlighted by Dr. Wulff-Burchfield:
- EvoPar PR01 (NCT06120491): for patients with mHSPC with or without deleterious HRR mutations who are randomized to systemic therapy standard of care + saruparib (PARP1 selective) versus systemic therapy standard of care + placebo
- STAMPEDE2 Stereotactic Ablative Body Radiotherapy Trial (NCT06320067): for patients with oligometastatic mHSPC without visceral metastatic disease who are randomized to systemic therapy standard of care versus systemic therapy standard of care + stereotactic ablative body radiotherapy to metastases
- STAMPEDE2 Niraparib-Abiraterone acetate + prednisolone (NCT06320067): for patients with mHSPC with a deleterious HRR mutation who are randomized to ADT + abiraterone acetate + prednisolone + niraparib versus ADT + physician’s choice of novel hormonal therapy
Dr. Wulff-Burchfield discussed clinical updates in mCRPC, starting with PSMAfore [4], which assessed whether we can use 177Lu-PSMA-617 before chemotherapy. Eligible adults for PSMAfore had mCRPC, were candidates for ARPI change after one progression on prior ARPI, and had ≥1 PSMA positive lesions and no exclusionary PSMA negative lesions by 68Ga-PSMA-11 PET/CT. Candidates for PARP inhibition and patients with prior systemic radiotherapy (<6 months ago), immunotherapy (except sipuleucel-T), or chemotherapy (except [neo]adjuvant >12 months ago) were ineligible. Randomization was 1:1 to open-label 177Lu-PSMA-617 (7.4 GBq every 6 weeks for 6 cycles) or ARPI change (abiraterone or enzalutamide). Importantly, patients randomized to ARPI could crossover to 177Lu-PSMA-617 following centrally reviewed radiographic progression. The trial design for PSMAfore is as follows:

At the time of the third data cutoff, the primary endpoint of radiographic progression free survival was met (HR 0.49, 95% CI 0.39 to 0.61), favoring the 177Lu-PSMA-617 arm:

For overall survival, there was no difference between the groups (HR 0.98, 95% CI 0.75-1.28), most likely secondary to 57.3% of patients crossing over in the ARPI change group and crossing over occurring among 77.5% of eligible patients:

CONTACT-02 was initially presented at ASCO GU 2024, randomizing patients 1:1 to cabozantinib + atezolizumab (cabozantinib [40 mg PO daily] + atezolizumab [1200 mg IV every 3 weeks]) or control (abiraterone [1000 mg PO daily] + prednisone [5 mg PO twice daily] or enzalutamide [160 mg PO daily]) and were stratified by liver metastasis (yes/no), prior docetaxel for mCSPC (yes/no), and prior novel hormonal therapy for mCSPC, M0CRPC, or mCRPC. The trial design for CONTACT-02 is as follows:

The median radiographic PFS was significantly longer with cabozantinib + atezolizumab vs control (6.3 vs 4.2 months; HR 0.65, 95% CI 0.50-0.84):

The final overall survival analysis, presented at ESMO 2024, did not demonstrate a survival benefit for cabozantinib + atezolizumab (median: 14.8 versus 15 months; HR 0.89, 95% CI 0.72–1.10, p = 0.30):

Dr. Wulff-Burchfield noted one key upcoming trial in the mCRPC disease space, which is a study of JNJ-69086420, an actinium-225 labelled antibody targeting human kallikrein-2 (hK2) for advanced prostate cancer (NCT04644770). This trial is for patients with mCRPC who have had treatment with one prior APRI, with the phase 2/expansion cohort being a single arm trial evaluating the phase 1 dose for safety and efficacy.
Dr. Wulff-Burchfield concluded her presentation discussing clinical trial updates for treatment of metastatic prostate cancer with the following take-home points:
- More is more: EMBARK, ARANOTE Darolutamide > placebo
- Sooner is better: UpFrontPSMA, PSMAfore
- Different is nice, but it sure isn’t pretty: CONTACT-02, PATCH
Presenter: Elizabeth Wulff-Burchfield, MD, University of Kansas School of Medicine, Kansas City, KS
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 South Central American Urological Association (AUA) Annual Meeting, Colorado Springs, CO, Wed, Oct 30 – Sat, Nov 2, 2024.
References:
- Freedland SJ, de Almeida Luz M, De Giorgi U, et al. Improved Outcomes with Enzalutamide in Biochemically Recurrent Prostate Cancer. N Engl J Med 2023 Oct 19;389(16):1453-1465.
- Saad F, Vjaters E, Shore N, et al. Darolutamide in Combination with Androgen-Deprivation Therapy in Patients with Metastatic Hormone-Sensitive Prostate Cancer From the Phase III ARANOTE Trial. J Clin Oncol. 2024 Sep 16 [Epub ahead of print].
- Azad AA, Bressel M, Tan H, et al. Sequential [(177)Lu]Lu-PSMA-617 and docetaxel versus docetaxel in patients with metastatic hormone sensitive prostate cancer (UpFrontPSMA): A multicentre, open label, randomized, phase 2 study. Lancet Oncol. 2024 Oct;25(10):1267-1276.
- Morris MJ, Castellano D, Herrmann K, et al. 177Lu-PSMA-617 versus a change of androgen receptor pathway inhibitor therapy for taxane-naïve patients with progressive metastatic castration-resistant prostate cancer (PSMAfore): A phase 3, randomized, controlled trial. Lancet 2024 Sep 28;404(10459):1227-1239.


