(UroToday.com) The 2024 South Central AUA annual meeting included a session on bladder cancer, featuring a presentation by Dr. Neema Navai discussing emerging treatments for non muscle invasive bladder cancer (NMIBC).
From a historical perspective, Dr. Navai notes that Dr. Raymond Pearl performed a case control autopsy study (controlling for sex, age, and race) in 1929 assessing the rate of tuberculosis, finding that in cancer patients it was 6.6% compared to 16.3% in non-cancer patients. Based on these results, he hypothesized that tuberculosis may be protective for cancer. Fast forward to 1976, when Dr. Morales and colleagues published the first report of BCG in bladder cancer among 7 patients: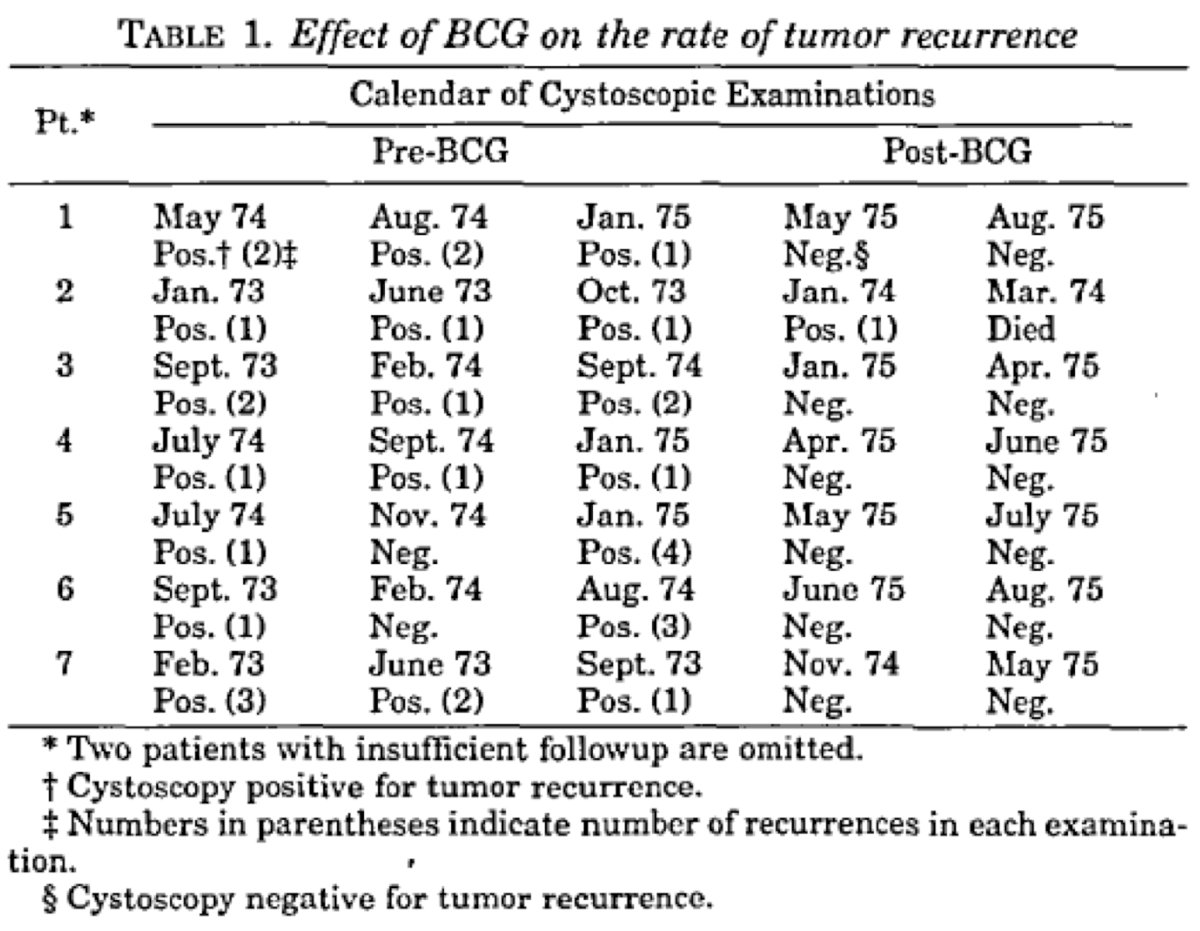
It would take till 2000 for the SWOG trial to demonstrate that maintenance BCG after induction therapy led to improved outcomes compared to no maintenance therapy:1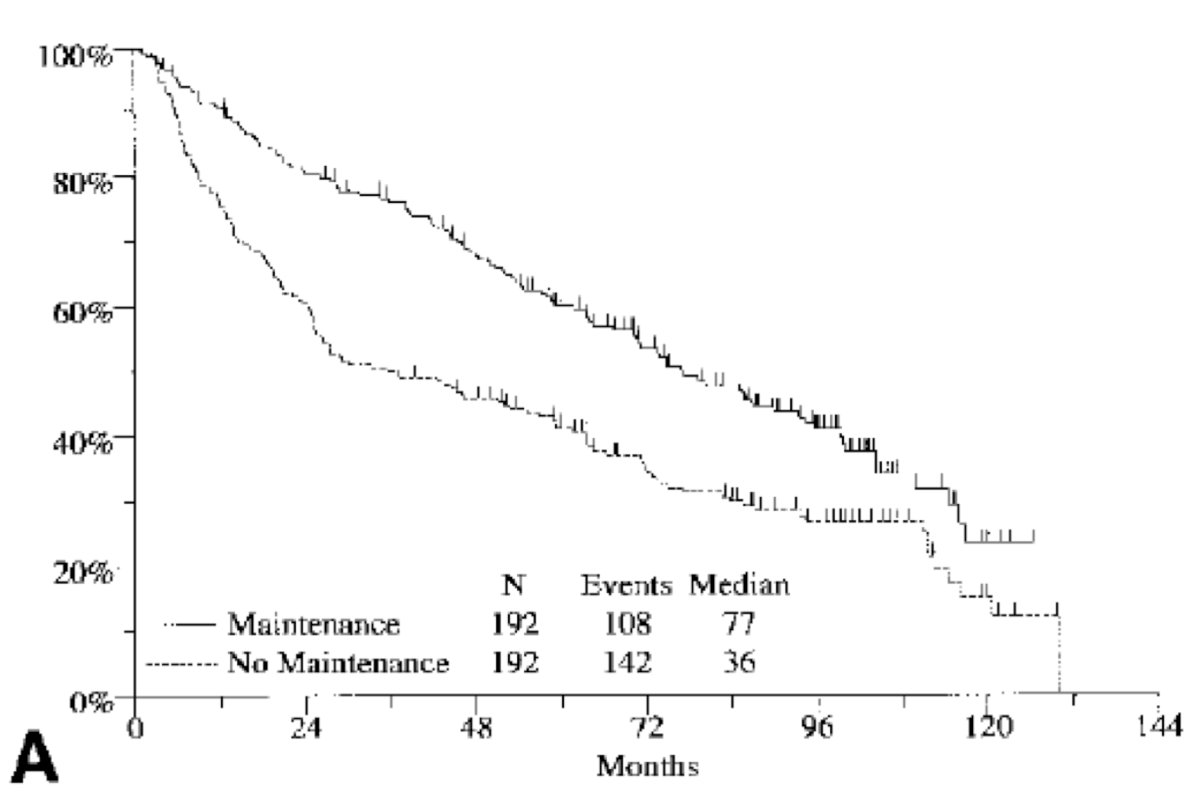
In a more contemporary cohort of patients, Matulay et al.2 retrospectively reviewed patients receiving adequate BCG therapy at MD Anderson Cancer Center between January 2004 and August 2018. Among 542 patients who received adequate BCG, 518 (90%) had EAU high risk disease, with CIS present in 175 (32%) specimens. Over a median follow up of 47.8 months, freedom from high grade recurrence at 1, 3, and 5 years was 81%, 76%, and 74%, respectively, and progression-free survival was 97%, 93% and 92%, respectively. Progression to muscle invasion at 5 years was exclusively seen in patients with high risk disease (progression-free survival 91%; log-rank test, p = 0.024):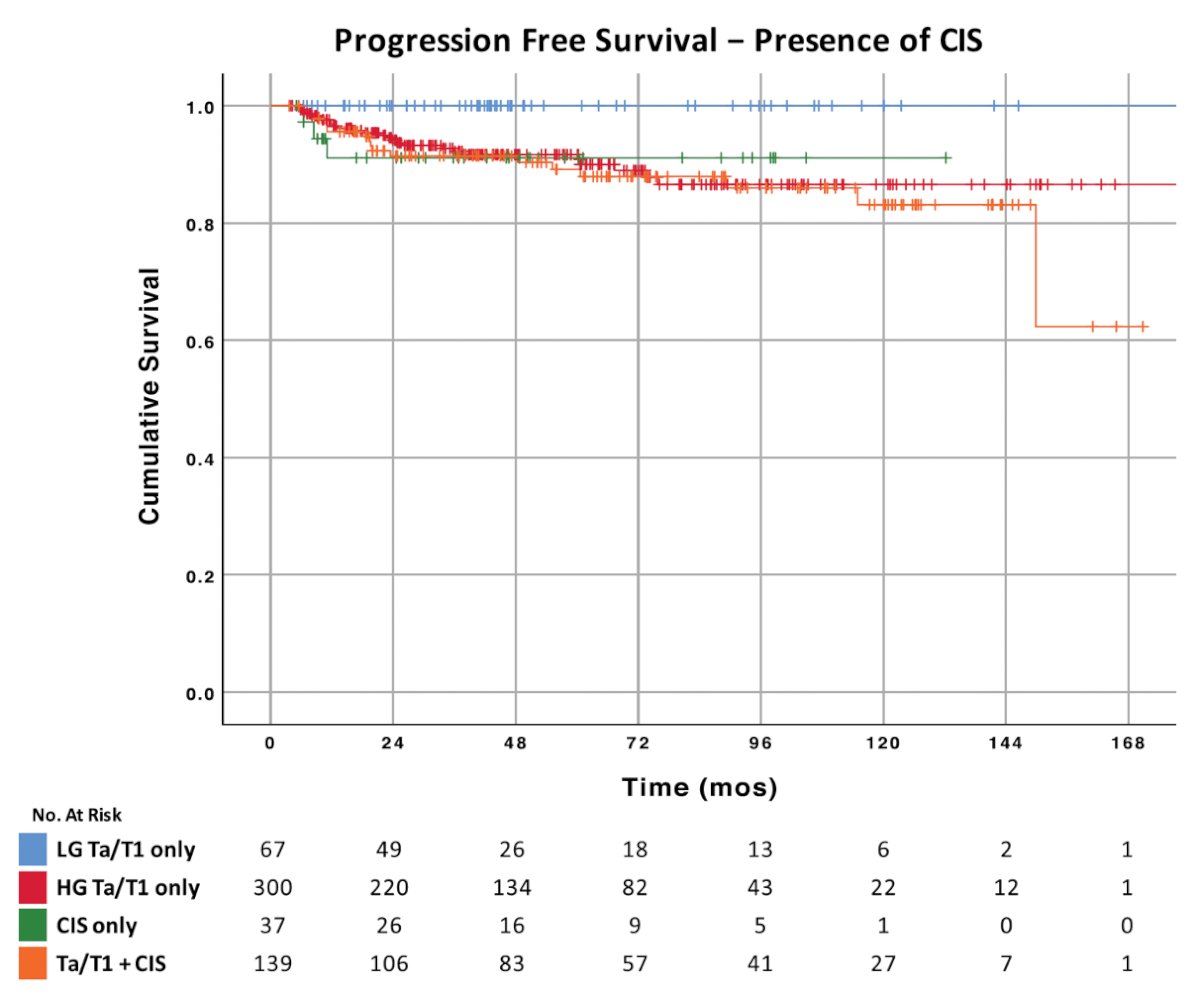
Additional work from Dr. Navai’s group at MD Anderson published in 2021 showed the importance of why disease progression after BCG matters.3 Among 801 patients who underwent radical cystectomy, 20.3% had progressive muscle invasive bladder cancer and 79.7% had de novo muscle invasive bladder cancer. In low-risk patients treated without neoadjuvant chemotherapy, progressive muscle invasive bladder cancer was associated with pathological upstaging (64.9% vs 42.7%, p = 0.004), as well as worse overall survival (p = 0.006) and cancer-specific survival (p = 0.001) compared to de novo muscle invasive bladder cancer: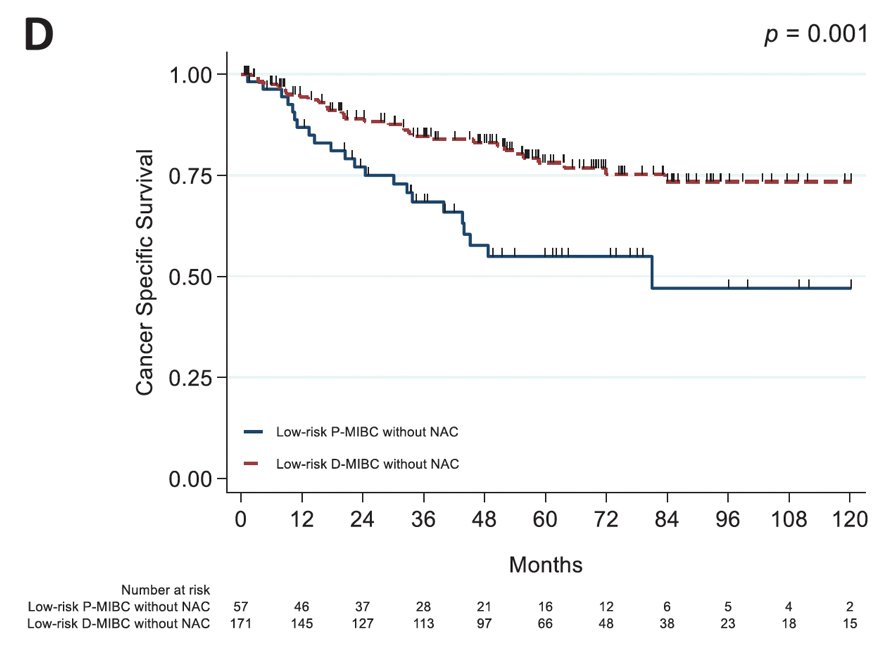
Dr. Navai notes that there are several reasons to move past BCG, highlighted as follows:
- Shortages have become untenable
- Therapy is extremely burdensome
- Tolerability can be an issue
- There are growing contraindications (ie. anti-TNF)
- Salvage/post BCG setting may identify more impactful front-line therapy
- We are getting bored and like new treatment options
Importantly, there are several clinical scenarios where the importance of additional therapies beyond BCG are important:
- cTa low grade, but multiple high volume recurrences despite negative upper tract imaging and enhanced cystoscopic resection followed by intravesical therapy with one year of maintenance
- High volume cT1/cTIS, refusing cystectomy and refractory disease after induction BCG
- cTa high grade recurrence 9 months after induction + maintenance BCG
- cTis in a patient who experienced BCG sepsis during induction BCG
- cTa/cTis in a patient 18 months after induction + maintenance BCG
Dr. Navai then discussed several trials and agents as alternatives to BCG. The KEYNOTE-057 trial4 assessed pembrolizumab for treatment of patients with BCG-unresponsive high-risk non-muscle invasive bladder cancer with CIS with or without papillary tumors who are ineligible for or have elected to not undergo cystectomy. Based on the interim results from 96 patients in this open-label, single-arm, multicenter, phase II trial, the FDA approved pembrolizumab in January 2020. Over a median follow up of 28.4 months, among patients with CIS alone (64%), 40% had a complete response at 3 months, and among those patients 53% maintained a complete response greater than 9 months. Over a median follow-up of 24.3 months, the median duration of complete response was 16.2 months. However, the 1-year complete response rate was only 19%.
Next, Dr. Navai discussed vicinium, which is an antibody drug conjugate-like recombinant fusion protein of humanized anti-EpCAM antibody and Pseudomonas exotoxin A administered intravesically. Of note, EpCAM is overexpressed in >98% of high grade NMIBC. The antibody component binds to the cancer cell causing the internalization of the molecule. Once internalized it releases the cytotoxic exotoxin A, which induces apoptosis: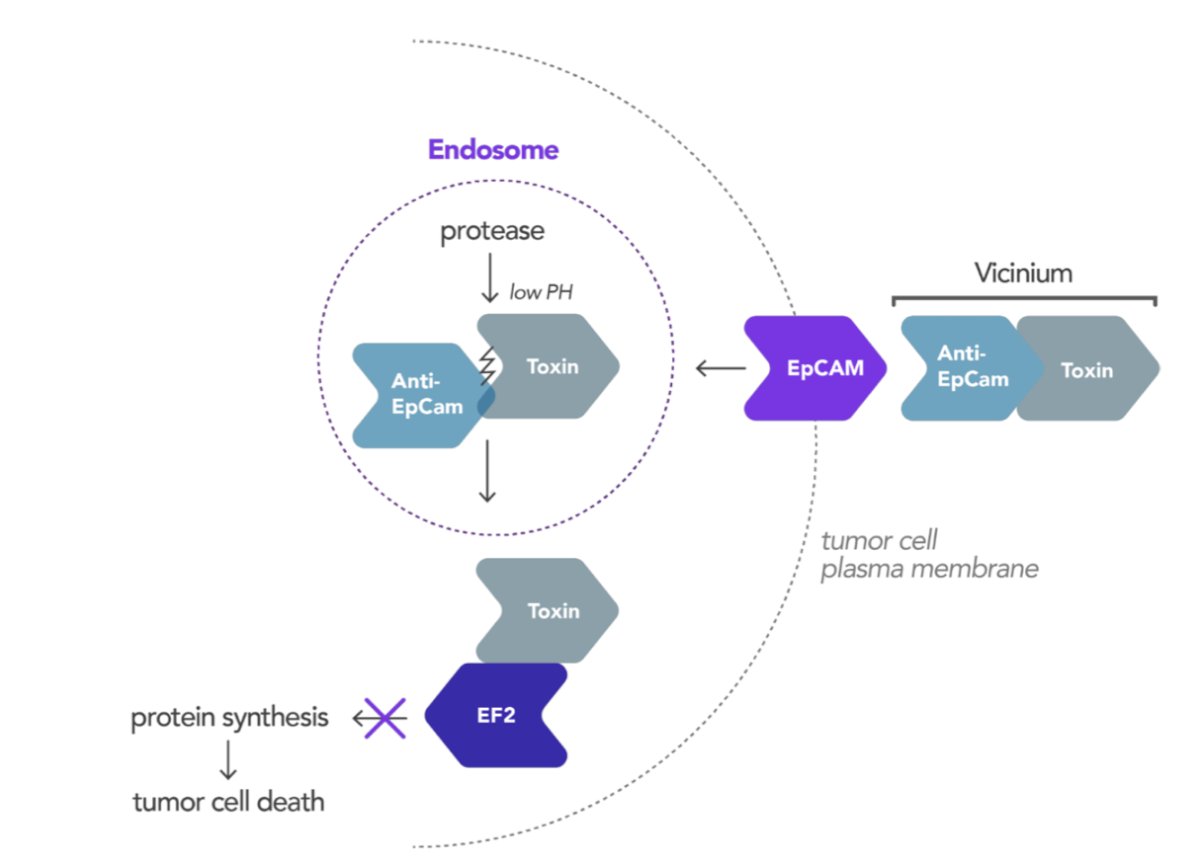
Unfortunately, development was paused after a reported 12 month complete response rate of 15.6%.
Next, Dr. Navai discussed the importance of the FGFR receptor. The FGF/FGFR signaling pathway plays a role in many physiologic process, including embryogenesis, adult tissue hemostasis, and wound healing. Alterations in FGFR signaling play a critical role in cancer cell proliferation, migration angiogenesis, and survival. FGFR signaling may be deregulated through alterations, including gene amplification, mutation, or translocation. Importantly, FGFR alterations are observed in 40-80% of NMIBC and 20% of muscle invasive bladder cancer. FGFR3 inhibition has been established with erdafitinib (THOR-2), rogaratinib (NCT0404725 – withdrawn), dovitinib (NCT01732107 – terminated), infigratinib, and pemibatinib.
The first safety and efficacy results of the TAR-210 erdafitinib intravesical delivery system were presented at AUA 2024. The study was also divided into two parts:
- Part 1 was a dose escalation phase with two different erdafitinib release rates being evaluated
- Part 2 was the dose expansion phase for patients in Cohorts 1 and 3. Response was assessed every 3 months with continued treatment for up to 1 year if recurrence-free (Cohort 1) or until complete response (Cohort 3). Of note, the first response assessment was at 3 months
In cohort 1, 90% of patients were recurrence free at 12 months, the median recurrence free survival was not estimable, and the median duration of follow-up was 8.9 months. In cohort 3 only 321 patients were evaluable for response, 90% achieved a complete response rate, with 28/31 achieving a complete response at week 12. At time of data analysis, 84% (24/28) complete response were ongoing.
Also presented at AUA 2024, was results from the SunRISe-1 trial, a phase 2b trial of TAR-200 gemcitabine. The trial design for SunRISe-1 is as follows: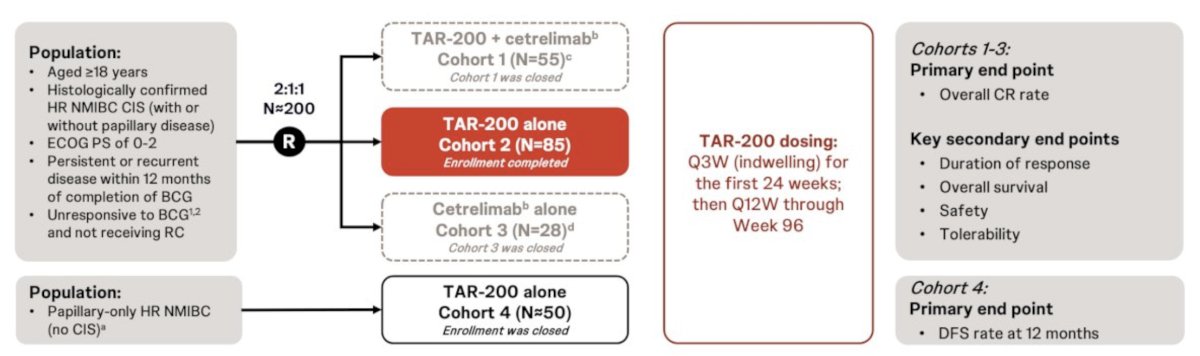
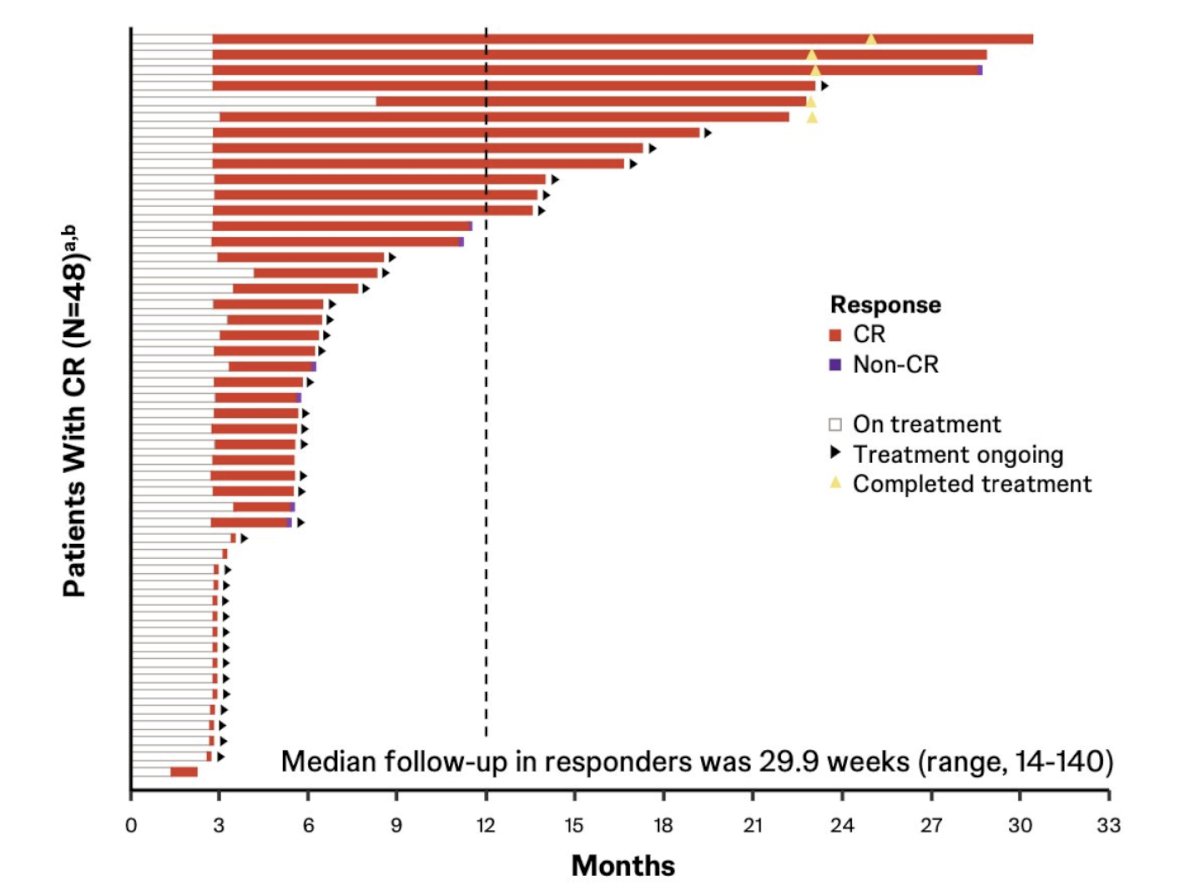
There was an 82.8% complete response rate via central review and an 86.2% complete response rate via investigator assessment, with the complete response rates at 6 and 12 months estimated to be 75.7% and 61.9%, respectively. TAR-200 was also associated with a durable response, with 41 of 48 (85%) responses ongoing at clinical cutoff, with 4 of 5 (80%) who completed 2-year treatment remaining in response. None of the responders progressed to muscle invasive bladder cancer or metastatic disease, and only 1 of 48 (2.1%) responders underwent a radical cystectomy. The Kaplan-Meier estimates for the duration of response:
- 6-month duration of response rate: 87.0% (95% CI, 69.0-94.9)
- 12-month duration of response rate: 74.6% (95% CI, 49.8-88.4)
- 18-month duration of response rate: 74.6% (95% CI, 49.8-88.4)
In the wake of the BCG shortage over the last several years, intravesical gemcitabine + docetaxel has emerged as a combination therapy for patients with NMIBC. In 2020, Steinberg et al.5 investigated intravesical gemcitabine/docetaxel as rescue therapy for NMIBC. Among 276 patients over a median follow-up of 22.9 months, 39% of patients were CIS alone, 26% were Ta high grade, 21% were T1 high grade, and 13% were Ta low grade. Overall, 53% had one BCG induction course, and 46% had 2+ BCG induction courses, and 38% were “BCG unresponsive.” Of note, some responders went on to maintenance therapy (monthly versus the SWOG schedule) for 24 months. One and 2-year recurrence-free survival rates were 60% and 46%, and high grade recurrence-free survival rates were 65% and 52%, respectively. Ten patients (3.6%) had disease progression on transurethral resection, and 43 patients (15.6%) went on to cystectomy (median 11.3 months from induction), of whom 11 (4.0%) had progression to muscle invasion.
Nadofaragene firadenovec-vncg, a non-replicating adenovirus vector-based gene therapy, is approved by the US FDA for patients with BCG-unresponsive non-muscle-invasive bladder cancer with CIS with/without papillary tumors (±Ta/T1).6 The primary endpoint of the open-label, multicenter, phase 3 study of nadofaragene firadenovec was met, as 53.4% (95% CI 43.3-63.3) of patients with CIS ± Ta/T1 achieved complete response at 3 months. Among patients with Ta/T1, there was a 73% complete response at 3 months, with a duration of response of 12.4 months, and a 12 month recurrence free survival rate of 44%.
BOND-003 is a phase 3 trial of cretostimogene monotherapy for BCG-unresponsive high-risk NMIBC with CIS. Cretostimogene Grenadenorepvec is a highly immunogenic conditionally replication adenovirus, and its oncolytic immunotherapy mechanism is that it encodes GM-CSF with insertion of the human EF2-1 promoter. The trial is a single-arm, open-label, intravesical administration of cretostimogene monotherapy for patients with pathologically confirmed BCG-unresponsive high-risk NMIBC with CIS +/- Ta/T1. Patients had all Ta/T1 disease resected prior to treatment, with mandatory biopsies at the 12-month assessment. The dosing regimen is an induction course of six weekly treatments, with the option for a second induction of six weekly treatments for non-responders. The endpoints for the trial are complete response at any time, complete response at 12 months, duration of response, progression free survival, and recurrence free survival. Among 116 patients enrolled, 66 were analyzed, including 82% with CIS only, 15% with CIS + Ta, and 3% with CIS + T1. With regards to treatment related adverse events, 56.3% had at least one event, there were no grade 3 events, no treatment discontinuation due to treatment related adverse events, and no deaths. Oncologic outcomes included a 76% complete response rate at any time, and a 64% response rate at 6 months. The conclusions from the BOND-003 trial are as follows:
- Cretostimogene is well tolerated and shows activity in BCG unresponsive patients
- Preliminary data are exciting, and we await more mature data
- Cretostimogene was granted breakthrough therapy and fast track designation by the FDA (expedited regulatory review)
CORE-001 is a phase 2 trial of cretostimogene + pembrolizumab for BCG unresponsive NMIBC CIS.7 Among 35 patients, the overall complete response rate was 85%, the complete response rate at 6 months was 82%, and the complete response rate at 12 months was 68%. There were transient grade 1/2 local symptoms, as well as tolerable grade 3 immune related safety consistent with prior anti-PD1 checkpoint inhibitor studies.
Finally, Dr. Navai discussed the Quilt 3032 trial.8 N-803 (nogapendekin alfa inbakicept: ANKTIVA®), is an interleukin-15 superagonist (IL-15), that promotes activation and proliferation of natural killer cells, CD8+ T cells and memory T cells without expanding immunosuppressive T-reg cells. N-803 synergizes with BCG to elicit durable complete responses and has recently been FDA-approved for BCG-unresponsive NMIBC CIS, with or without papillary tumors. In this trial, over a median follow up of 23.9 months, the complete response rate was 71% and the median duration of response was 26.6 months. Based on these results, the FDA granted breakthrough approval of N-803 in April 2024.
Dr. Navai concluded his presentation discussing emerging treatments for NMIBC by noting that there are several additional ‘honorable mention’ options after BCG including intravesical enfortumab vedotin, nab-rapamycin + gemcitabine, and everolimus + gemcitabine. Combinations and prolonged delivery systems are likely the largest area of growth in this disease space.
Presented by: Neema Navai, MD, Associate Professor, The University of Texas, MD Anderson Cancer Center, Houston, TX
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 South Central American Urological Association (AUA) Annual Meeting, Colorado Springs, CO, Wed, Oct 30 – Sat, Nov 2, 2024.
References:
- Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell of the bladder: A randomized Southwest Oncology Group Study. J Urol. 2000;163:1124-1129.
- Matulay JT, Li R, Hensley PJ, et al. Contemporary Outcomes of Patients with Nonmuscle-Invasive Bladder Cancer Treated with bacillus Calmette-Guérin: Implications for Clinical Trial Design. J Urol. 2021;205(6):1612-1621
- Hensley PJ, Bree KK, Campbell MT, et al. Progression of disease after Bacillus Calmette-Guerin Therapy: Refining Patient Selection for Neoadjuvant Chemotherapy before Radical Cystectomy. J Urol. 2021 Nov;206(5):1258-1267.
- Balar AV, Kamat AM, Kulkarni GS, et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): An open-label, single-arm, multicenter, phase 2 study. Lancet Oncol. 2021 Jul;22(7):919-930.
- Steinberg RL, Thomas LJ, Brooks N, et al. Multi-institution evaluation of sequential gemcitabine and docetaxel as rescue therapy for nonmuscle invasive bladder cancer. J Urol. 2020;203:902-909.
- Boorjian SA, Alemozaffar M, Konety BR, et al. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: A single-arm, open-label, repeat-dose clinical trial. Lancet Oncol. 2021 Jan;22(1):107-117.
- Li R, Shah PH, Stewart TF, et al. Oncolytic adenoviral therapy plus pembrolizumab in BCG-unresponsive non-muscle-invasive bladder cancer: The phase 2 CORE-001 trial. Nat Med. 2024 Aug;30(8):2216-2223.
- Chamie K, Chang SS, Kramolowsky E, et al. IL-15 Superagonist NAI in BCG-Unresponsive Non-Muscle-Invasive Bladder Cancer. NEJM Evid 2022; 2(1).


