(UroToday.com) The 2024 South Central AUA annual meeting included a session on bladder cancer, featuring a presentation by Dr. Cheryl Lee discussing the top 10 reasons why BCG as a monotherapy will cease to exist.
#10: There are challenges of administration. This includes general side effects, intolerance to BCG in ~5% of patients leading to discontinuation, dwell time challenges, and the treatment intensity it takes to get through induction + a long course of maintenance therapy.
#9: BCG will face increased competition in the intermediate risk NMIBC disease space. The recently published ENVISION trial1 is an ongoing, multinational, single-arm, phase 3 study in patients with a biopsy-proven recurrence of untreated low-grade intermediate-risk NMIBC. Patients received 6 weekly intravesical instillations of UGN-102 (outpatient setting) and were evaluated at 3 months. Of 240 patients enrolled, 228 (95%) received all 6 planned doses, and 191 (79.6%; 95% CI: 73.9, 84.5) achieved complete response at 3 months, with an 82.3% (95% CI: 75.9, 87.1) probability of response 12 months later. Median duration of response was not estimable over a median 13.9-month follow-up period.
#8: A targeted agent will drive precision care. THOR-2 Cohort 12 evaluated the activity of oral erdafitinib, a selective pan-FGFR tyrosine kinase inhibitor, versus intravesical chemotherapy in patients with high-risk NMIBC and select FGFR3/2 alterations following recurrence after BCG treatment. There were 73 patients randomized 2:1 to erdafitinib (n = 49) and chemotherapy (n = 24). Over a median follow-up for recurrence free survival of 13.4 months in both groups, the median recurrence free survival was not reached for erdafitinib (95% CI 16.9 months-NE) and was 11.6 months (95% CI 6.4-20.1 months) for chemotherapy (HR 0.28, 95% CI 0.10-0.60):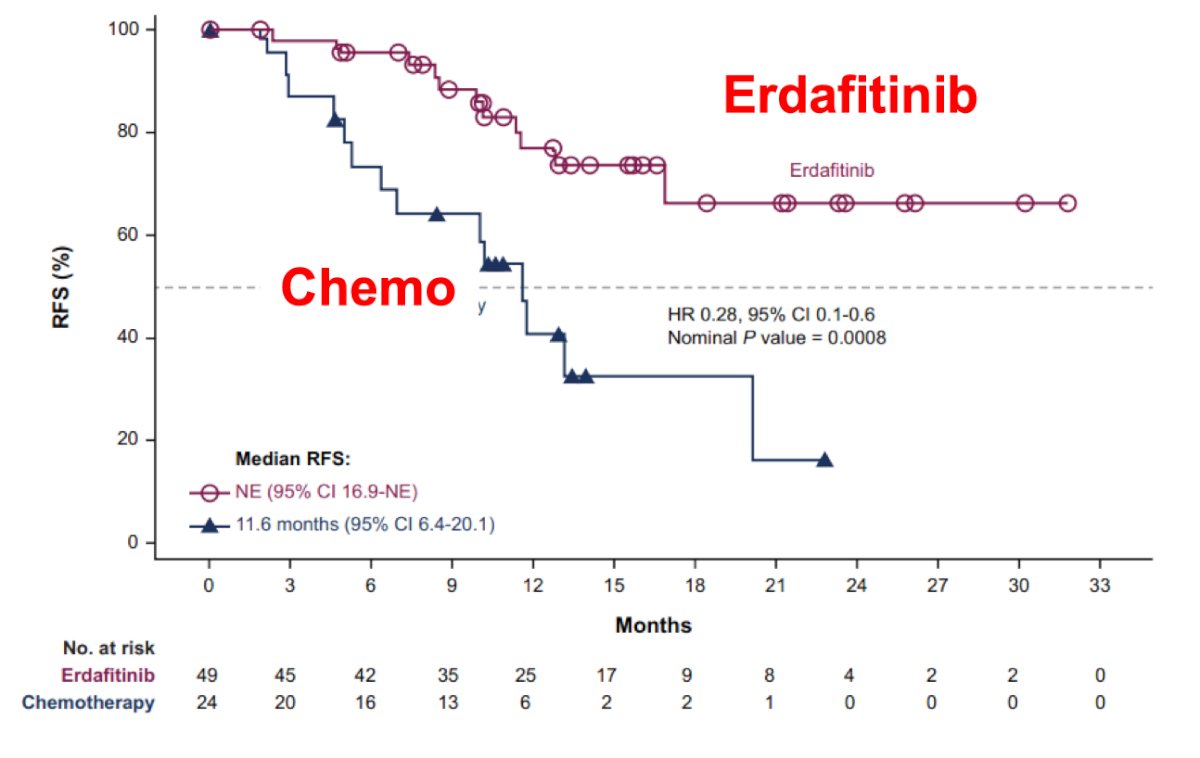
#7: Novel agents are improving drug delivery over BCG. Updated results from the SunRISe-1 trial, a phase 2b trial of TAR-200 gemcitabine, were presented at ESMO 2024. The trial design for SunRISe-1 is as follows: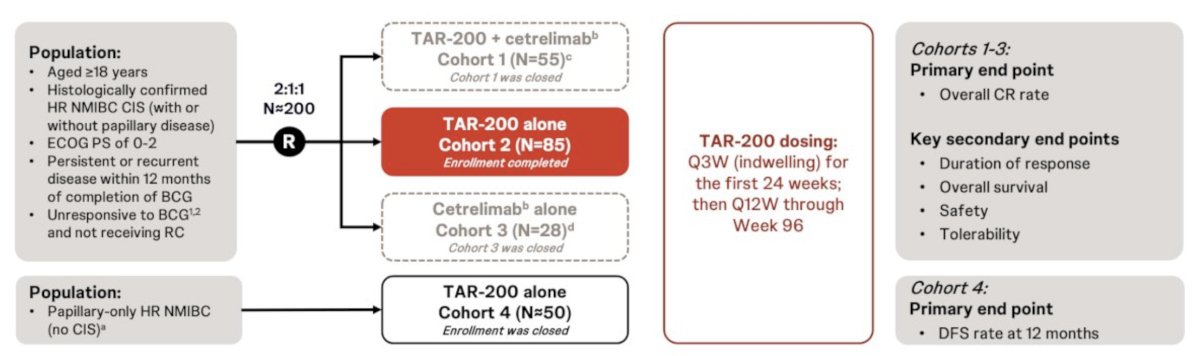
Complete response rate by investigator assessment was comparable with central results (80.0% and 76.7%):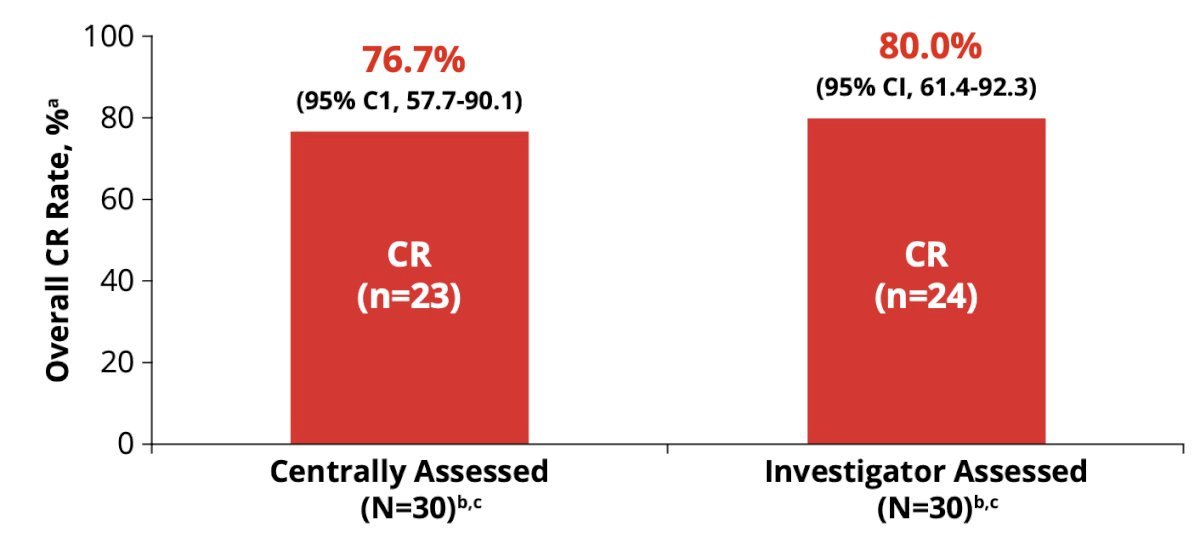
#6: Novel agents have more convenient treatment schedules than BCG. Notably, nadofaragene firadenovec, is a non-replicating adenovirus vector-based gene therapy and approved by the US FDA for patients with BCG-unresponsive NMIBC with CIS with/without papillary tumors (±Ta/T1).3 The treatment schedule is intravesical delivery every 3 months for 5 doses. The primary endpoint of the open-label, multicenter, phase 3 study of nadofaragene firadenovec was met, as 53.4% (95% CI 43.3-63.3) of patients with CIS ± Ta/T1 achieved complete response at 3 months. Among patients with Ta/T1, there was a 73% complete response at 3 months, with a duration of response of 12.4 months, and a 12 month recurrence free survival rate of 44%. Moreover, the 5-year cystectomy free survival rate is 43% for CIS ± Ta/T1 and 59% for Ta/T1.
#5: Novel agents are synergistic with BCG. The QUILT-3.032 trial4 assessed N-803 (nogapendekin alfa inbakicept: ANKTIVA®), an interleukin-15 superagonist (IL-15) that promotes activation and proliferation of natural killer cells, CD8+ T cells and memory T cells without expanding immunosuppressive T-reg cells. N-803 synergizes with BCG to elicit durable complete responses and has recently been FDA-approved for BCG-unresponsive NMIBC CIS, with or without papillary tumors. In this trial, over a median follow up of 23.9 months, the complete response rate was 71% and the median duration of response was 26.6 months: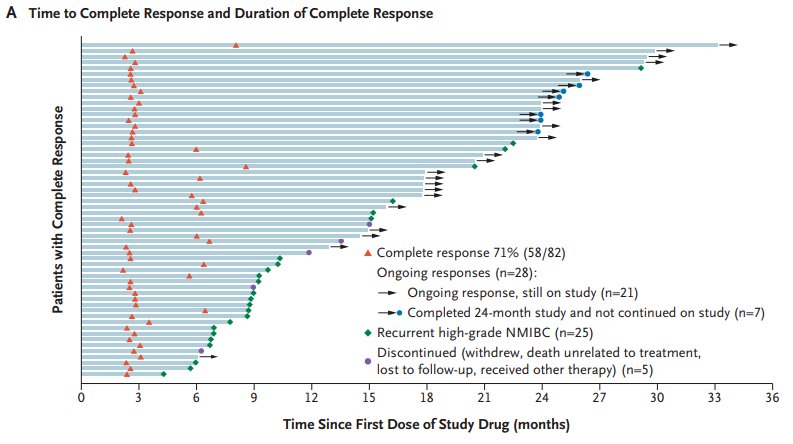
#4: There is a worldwide BCG shortage. In 2012, Merck became the sole manufacturer of TICE BCG and they are committed to build a new production facility in Durham, NC (opening in 2025-2026). This is crucial, as many urologists have limited access, which means that patients have lost access to BCG.
#3: Chemotherapy is challenging BCG as a front line agent for high risk NMIBC. Dr. Lee mentioned the BRIDGE trial (ECOG-ACRIN EA8212) which is evaluating gemcitabine + docetaxel versus BCG in the BCG-naïve setting: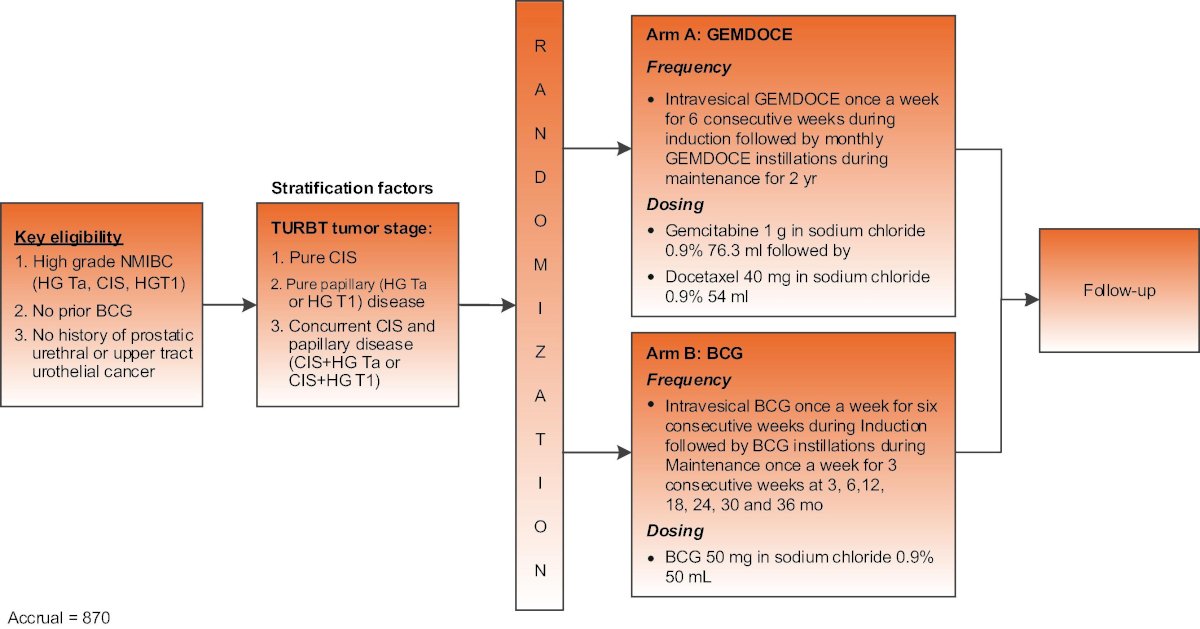
The accrual goal for this trial is 870 participants, with a primary endpoint of event free survival.
#1 and #2: Combination BCG + immunotherapy trials are ongoing as frontline treatment. This includes the following phase 3 trials of PD-1/PD-L1 inhibitors in BCG naïve high risk NMIBC: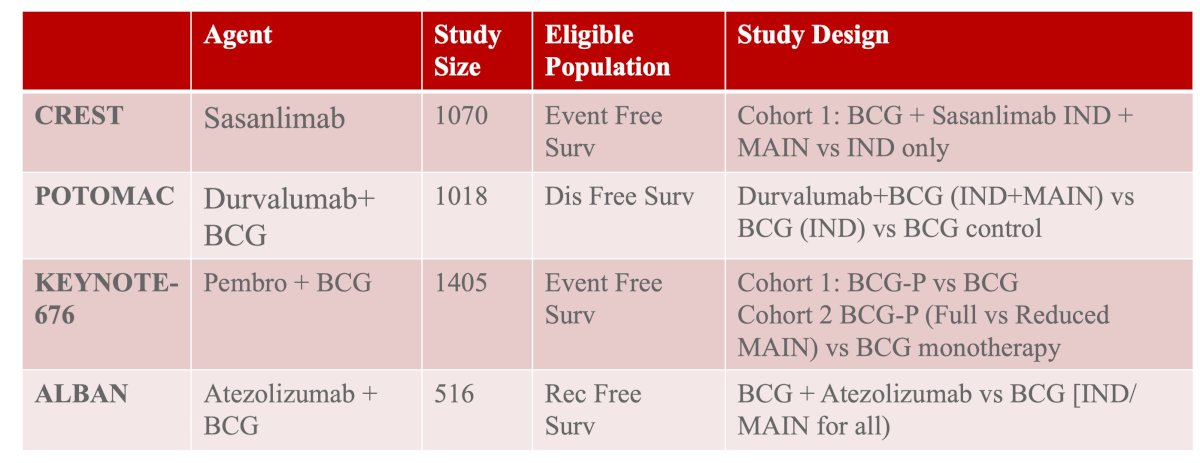
Dr. Lee concluded her presentation discussing the top 10 reasons why BCG as a monotherapy will cease to exist with the following take-home points:
- Several agents are competing with BCG as first line therapy in BCG naïve patients
- BCG is being studied in combination with various immunologic agents
- There is additional focus on reducing treatment burden for patients
- BCG, as a monotherapy, is likely to wane over the next 5 years as new data emerges
Presented by: Cheryl Lee, MD, Urology Specialist, Ohio State University, Columbus, OH
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 South Central American Urological Association (AUA) Annual Meeting, Colorado Springs, CO, Wed, Oct 30 – Sat, Nov 2, 2024.
References:
- Prasad SM, Shishkov D, Vladimirov Mihaylov N, et al. Primary Chemoablation of Recurrent Low-Grade Intermediate-Risk Nonmuscle-Invasive Bladder Cancer with UGN-102: A Single-Arm, Open-Label, Phase 3 Trial (ENVISION). J Urol. 2024 Oct 24 [Epub ahead of print].
- Catto JWF, Tran B, Roupret M, et al. Erdafitinib in BCG-treated high-risk non-muscle invasive bladder cancer. Ann Oncol. 2024;35(1):98-105.
- Boorjian SA, Alemozaffar M, Konety BR, et al. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: A single-arm, open-label, repeat-dose clinical trial. Lancet Oncol. 2021 Jan;22(1):107-117.
- Chamie K, Chang SS, Kramolowsky E, et al. IL-15 Superagonist NAI in BCG-Unresponsive Non-Muscle-Invasive Bladder Cancer. NEJM Evid 2022; 2(1).


