(UroToday.com) The 2024 South Central AUA annual meeting included a session on bladder cancer, featuring a presentation by Dr. Chad LaGrange discussing the case for radical cystectomy as treatment for high risk non muscle invasive bladder cancer (NMIBC). According to the AUA guidelines, high grade urothelial carcinoma has the following features:
- CIS, or
- T1, or
- >3 cm, or
- Multifocal
Very high risk features, include any of the following:
- BCG unresponsive
- Variant histologies
- Lymphovascular invasion
- Prostatic urethral invasion
Based on the NCCN guidelines, radical cystectomy is the preferred option for BCG naïve patients with very high risk features, as well as for BCG unresponsive or intolerant patients: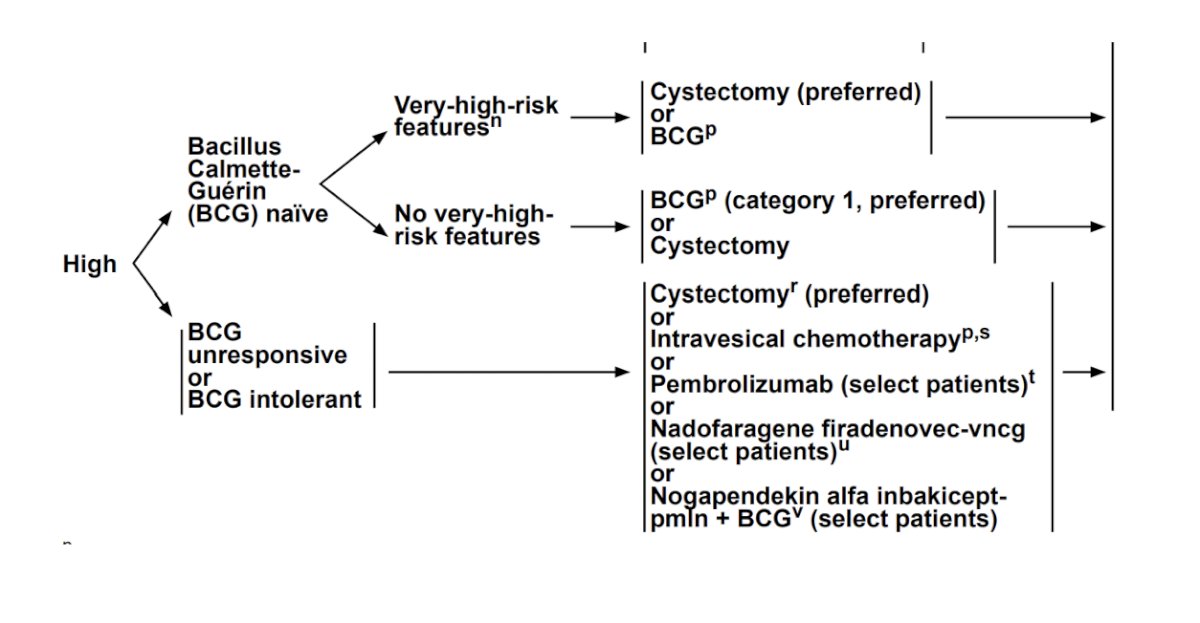
In Dr. LaGrange’s opinion, the ideal patients for radical cystectomy in the setting of NMIBC are those that are young and healthy, and/or have high volume CIS, and/or have large, multifocal disease burden. Looking closer at radical cystectomy for high risk NMIBC, Dr. LaGrange discussed the BRAVO trial,1 which attempted to randomize patients to radical cystectomy versus intravesical BCG for high-risk NIMBC. This trial was a two-arm, prospective multicenter randomized study to determine the feasibility in BCG-naive patients, in which patients had new high-risk NMIBC suitable for both treatment arms. There were 407 patients screened, of which 185 were approached for the trial, and consent was obtained from 51 (27.6%) patients. In the BCG arm, 23/25 (92.0%) patients received BCG, of which four had NMIBC after induction, three had NMIBC at 4 months, and four received radical cystectomy. In the radical cystectomy arm, 20 (80.0%) participants received cystectomy, including five (25.0%) with no tumor, 13 (65.0%) with high-risk NMIBC, and two (10.0%) with muscle invasion in their specimen. Of note, all patients in the radical cystectomy arm were disease free at 12 months.
Real world outcomes among patients screened for the BRAVO trial were recently published.2 Among 193 patients, this included 106 (54.9%) who received BCG, 43 (22.3%) received primary radical cystectomy, 37 (19.2%) had 'other' treatment, and seven (3.6%) received hyperthermic intravesical mitomycin C. All-cause death occurred in 55 (28.5%) patients at a median of 29.0 (IQR 19.5-42.0) months. In multivariable analysis, overall mortality was more common in older patients (HR 2.63, 95% CI 1.35-5.13; for age >70 years), those recruited from district hospitals (HR 0.53, 95% CI 0.3-0.95) and those who did not undergo radical cystectomy as their first treatment (HR 2.16, 95% CI 1.17-3.99).
Dr. LaGrange emphasized that variant histology is a particularly aggressive phenotype that should be managed with upfront radical cystectomy. These may include mixed (urothelial + variant): squamous, adenocarcinoma, micropapillary, nested, plasmacytoid, and sarcomatoid, or they may be pure squamous, adenocarcinoma, small cell/neuroendocrine, or sarcoma.
Regarding CIS, with treatment, 30-40% of CIS progresses to invasive disease within 10 years. Ideal candidates for radical cystectomy are those with (i) high volume CIS, (ii) CIS of the prostatic urethra, or (iii) CIS refractory to BCG. BCG refractory CIS at the time of radical cystectomy is upstaged >50% of the time, 25% have T2 disease, 5.8% have N+ disease, and cancer specific survival is 85%. For recurrent high grade T1, previous studies have demonstrated that 5-year death from disease decreased from 48% to 31% with immediate cystectomy versus other bladder sparing options.3 To conclude, Dr. LaGrange noted that lymphovascular invasion is associated with understaging, recurrence, and disease progression. Thus, those patients with high grade T1 + lymphovascular invasion are the most likely to benefit from early cystectomy.
Presented by: Chad A. LaGrange, MD, FACS, University of Nebraska, Omaha, NE
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 South Central American Urological Association (AUA) Annual Meeting, Colorado Springs, CO, Wed, Oct 30 – Sat, Nov 2, 2024.
References:- Catto JWF, Gordon K, Collinson M, et al. Radical Cystectomy Against Intravesical BCG for High-Risk High-Grade Nonmuscle Invasive Bladder Cancer: Results from the Randomized Controlled BRAVO-Feasibility Study. J Clin Oncol. 2021 Jan 20;39(3):202-214.
- Conroy S, Jubber I, Noon AP, et al. Real-world outcomes for high-risk non-muscle-invasive bladder cancer: screened patients for the BRAVO trial. BJU Int. 2024 Sep 26 [Epub ahead of print].
- Raj GV, Herr H, Serio AM, et al. Treatment paradigm shift may improve survival of patients with high risk superficial bladder cancer. J Urol. 2007;177:1283-1286.


