(UroToday.com) The 2024 South Central AUA annual meeting included a session on kidney cancer, featuring a presentation by Dr. Tyler Robin discussing indications and outcomes for radiation therapy in renal masses. Dr. Robin started his presentation by highlighting the current state of the data for stereotactic ablative radiotherapy for primary renal cell carcinoma.
In 2023, the International Radiosurgery Consortium of the Kidney (IROCK) group published an individual patient data meta-analysis of the 5 year outcomes after for stereotactic ablative radiotherapy for primary renal cell carcinoma.1 For this analysis, individual patient data from 12 institutions from IROCK were pooled. Patients with M1 disease and/or upper tract urothelial carcinoma were excluded, and minimum eligible follow-up was ≥ 2 years. Local failure was investigator defined using RECIST 1.1. Patterns of failure were described using a cumulative incidence function with death as a competing event, and toxicity was described using CTCAE v4.0.
In 190 patients, the median follow-up was 5.0 years (95% CI 4.6 – 5.2 years). The mean ± SD tumor diameter was 4.2 ± 2.2 cm and 95 patients (50%) had ≥ T1b (≥ 4 cm) primaries. Median age was 73.6 years (IQR: 66.2-82.0), 73.2% were male, and 87.6% had good performance status (ECOG 0-1 or KPS ≥ 70%). In patients for whom operability details were reported, 75.0% were defined as inoperable by the referring urologist, mostly for cardiovascular comorbidities (46.9%). Baseline tumor complexity was moderate (median RENAL score of 7 [IQR 5-9]), and 56 patients (29.5%) had a solitary kidney. The median biologically effective dose10 delivered was 87.5 Gy (range 33.5-180.0). Median baseline estimated GFR was 60.0 (IQR 42.0-76.0) mL/min (mild-to-moderate dysfunction) with 53 patients (28.0%) of the cohort having moderate-to-severe dysfunction (eGFR < 45 mL/min):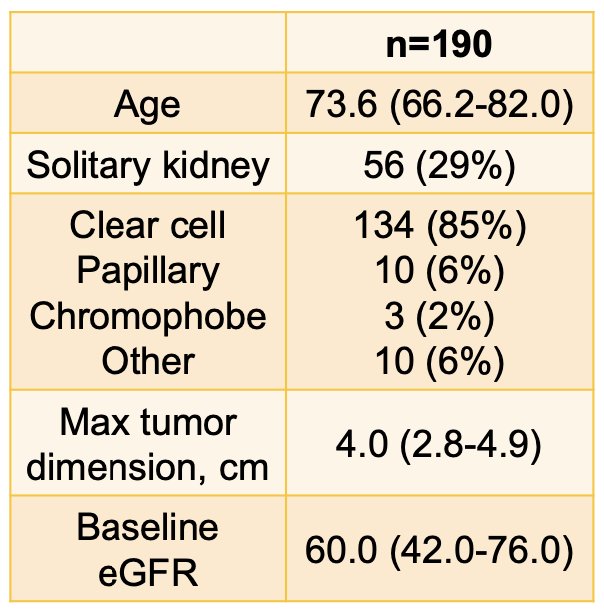
Seventy patients (37%) had a grade 1-2 toxicity, and one patient (1%) had a grade 4 gastrointestinal toxicity (at 1.4 months) and a grade 4 bowel toxicity (at 15.8 months; the patient is alive at 8.8 years without disease). The full adverse event profile is as follows: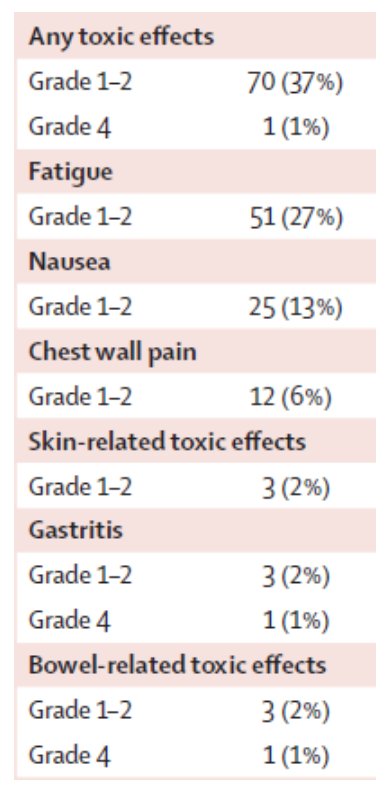
Local failure at 5 years was 5.5%: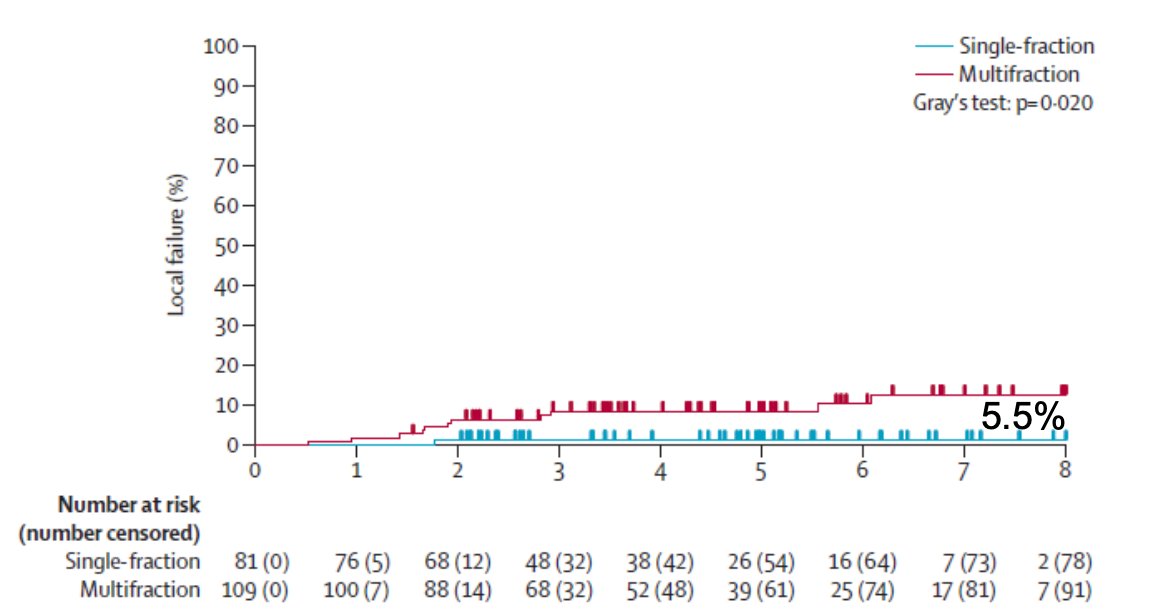
The TROG 15.03 FASTRACK II trial is a non-randomized phase 2 trial of stereotactic ablative body radiotherapy for primary kidney cancer, which was recently published in Lancet Oncology.2 There were 70 patients that received either a single fraction stereotactic ablative body radiotherapy of 26 Gy for tumours 4 cm or less in maximum diameter, or 42 Gy in three fractions for tumours more than 4 cm to 10 cm in maximum diameter. The primary endpoint was local control, defined as no progression of the primary renal cell cancer, as evaluated by the investigator per RECIST (version 1.1). The median age was 77 years (IQR 70-82), and before enrolment, 49 (70%) of 70 patients had documented serial growth on initial surveillance imaging. The median tumor size was 4.6 cm (IQR 3.7-5.5), and all patients enrolled had T1-T2a and N0-N1 disease. There were 23 patients that received single-fraction SABR of 26 Gy and 47 received 42 Gy in three fractions: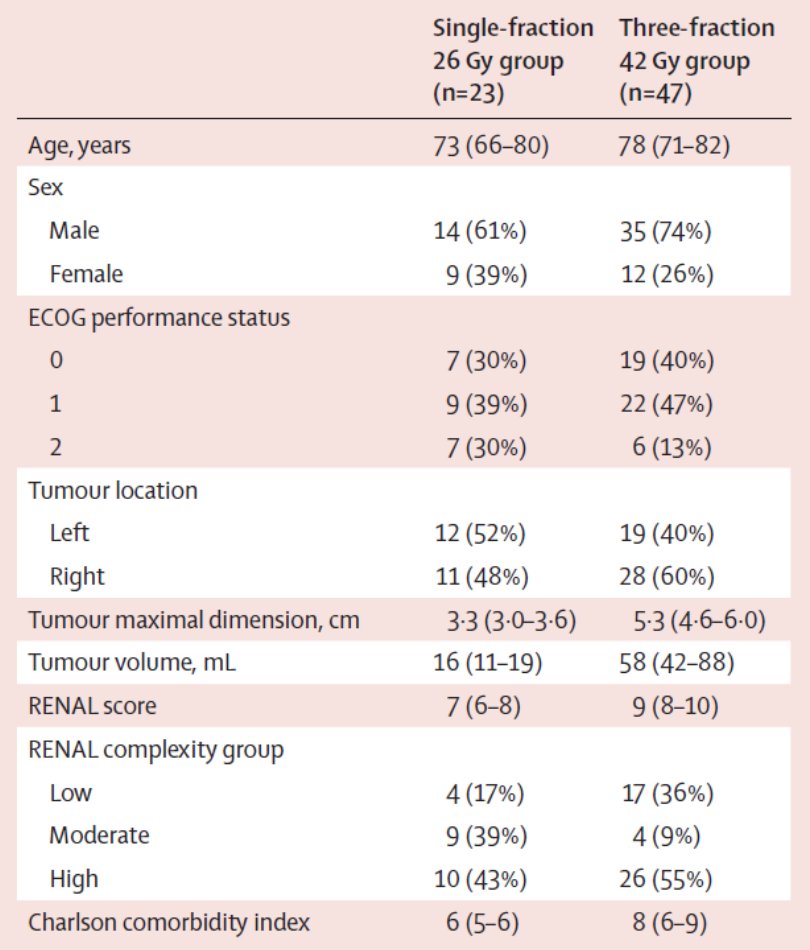
Median follow-up was 43 months (IQR 38-60), and local control at 12 months from treatment commencement was 100% (p < 0.0001):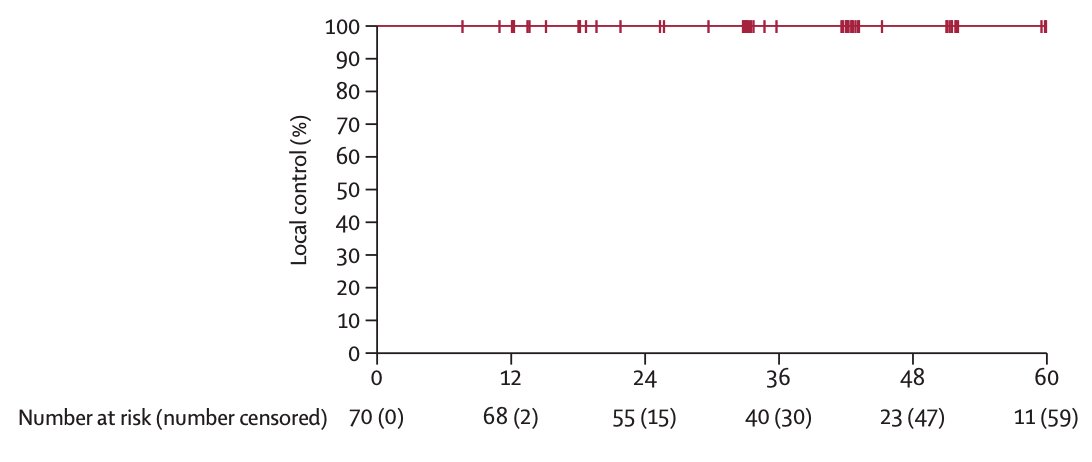
Seven (10%) patients had grade 3 treatment-related adverse events, with no grade 4 adverse events observed.
In 2024, Siva et al.3 published a systematic review and practice guideline assessing the body of literature for stereotactic body radiotherapy for primary renal cell carcinoma, which included 36 articles (23 retrospective, and 13 prospective; n = 822 patients). The median age was 72 years, 73% were male, median tumor diameter was 4.4 cm, biopsy confirmation was obtained in 98% of cases, and the median follow-up time was 31.2 months. The median local control rate was 94.1% (range 70.0-100), 5-year progression free survival was 80.5% (95% CI 72-92), and 5-year overall survival was 77.2% (95% CI 65-89).
Generally, several meta-analyses have demonstrated that partial nephrectomy, stereotactic body radiotherapy, and thermal ablation all have better eGFR outcomes compared to radical nephrectomy. With regards to post-treatment monitoring, there are several unique principles for monitoring after stereotactic body radiotherapy compared to after thermal ablation or partial nephrectomy. When assessing imaging, remember that:
- Reduction in tumor size by imaging is slow (occurs over years) after stereotactic body radiotherapy.
- Residual enhancement dose not predict local failure
Additionally, a positive biopsy is not uncommon after stereotactic body radiotherapy for RCC, despite high rates of local control. An analysis of post-treatment biopsies by Hannan et al.4 in a prospective phase 2 trial showed decreased cellularity, decrease Ki-67, increased fibrosis, and hyalinization, and increased markers of cellular senescence. Stereotactic body radiotherapy results in cell death in the majority of tumor cells, but a minority of cells enter senescence, or irreversible cell cycle arrest, and are not truly viable.
Dr. Robin concluded his presentation discussing indications and outcomes for radiation therapy in renal masses by highlighting the importance of patient selection for stereotactic body radiotherapy, which allows treatment of small peripheral tumors, larger tumors, tumors close to the hilum, and for patients that are medically inoperable/high risk: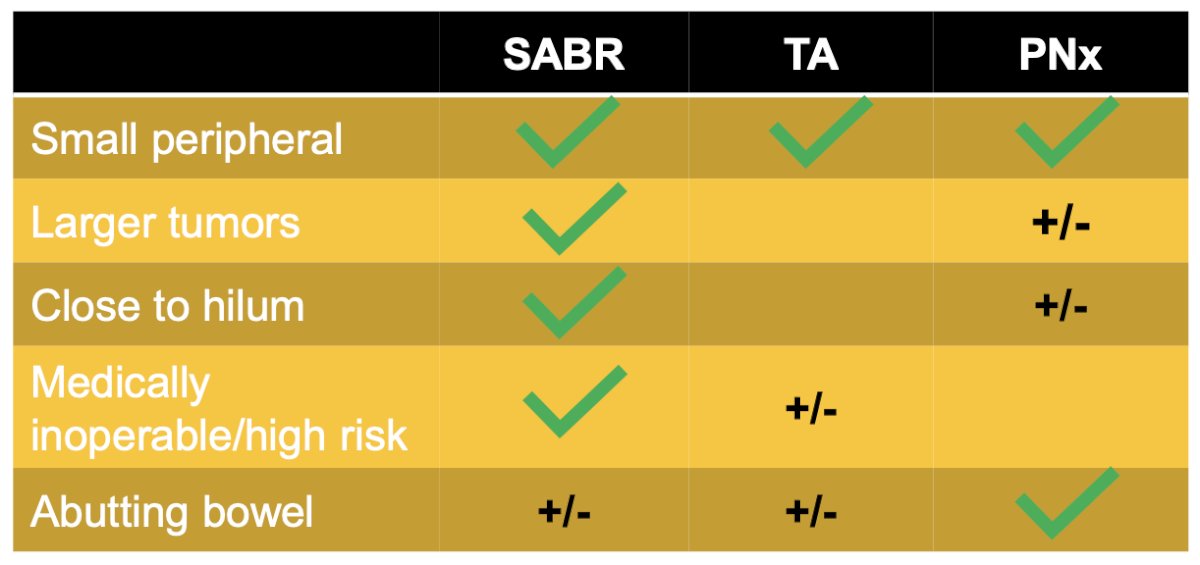
Presented by: Tyler Robin, MD, PhD, UC Health – Anschutz Medical Campus, Denver, CO
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 South Central American Urological Association (AUA) Annual Meeting, Colorado Springs, CO, Wed, Oct 30 – Sat, Nov 2, 2024.
References:
- Siva S, Ali M, Correa RJM, 5-year outcomes after stereotactic ablative body radiotherapy for primary renal cell carcinoma: An individual patient data meta-analysis from IROCK (the International Radiosurgery Consortium of the Kidney). Lancet Oncol. 2022 Dec;23(12):1508-1516.
- Siva S, Bressel M, Sidhom M, et al. Stereotactic ablative body radiotherapy for primary kidney cancer (TROG 15.03 FASTRACK II): A non-randomized phase 2 trial. Lancet Oncol. 2024 Mar;25(3):308-316.
- Siva S, Louie AV, Kotecha R, et al. Stereotactic body radiotherapy for primary renal cell carcinoma: A systematic review and practice guideline from the International Society of Stereotactic Radiosurgery (ISRS). Lancet Oncol. 2024 Jan;25(1):e18-e28.
- Hannan R, McLaughlin MF, Pop LM, et al. Phase 2 trial of stereotactic ablative radiotherapy for patients with primary renal cancer. Eur Urol. 2023 Sep;84(8):275-286.


