(UroToday.com) The 2024 South Central AUA annual meeting included a session on bladder cancer, featuring a presentation by Dr. Carlos Riveros discussing a systematic review and meta-analysis of randomized clinical trials assessing adjuvant immunotherapy in high-risk muscle invasive urothelial carcinoma. Approximately 50% of patients with muscle invasive urothelial carcinoma develop recurrence following radical surgery.
Adjuvant immune checkpoint inhibition following radical surgical resection in patients with muscle invasive disease demonstrates disparate outcomes among phase III randomized controlled trials. In light of extended follow up of CheckMate-2741 and recent results from the AMBASSADOR trial,2 Dr. Riveros and colleagues performed a systematic review to assess the summative disease free survival benefit, adverse event profile, and examine potential subgroups of clinical interest.
To synthesize recent data regarding the disease free survival benefit within muscle invasive urothelial carcinoma, Dr. Riveros and colleagues performed a systematic search and identified phase III randomized controlled trials comparing adjuvant immune checkpoint inhibition versus placebo/observation. Using terms “adjuvant” and “immunotherapy” and “urothelial carcinoma”, they searched MEDLINE, Embase, CENTRAL, and relevant conference proceedings. The primary and secondary outcomes of interest were disease-free survival and serious adverse events. A priori defined subgroups of interest included positive PD-L1 expression, previous use of neoadjuvant chemotherapy, primary tumor origin, pathologic lymph node status, and baseline ECOG status.
Three randomized controlled trials (n = 2,220 patients) were included, with all studies including disease free survival as the primary outcome, with treatment duration being 12 months. Inclusion of upper tract muscle invasive disease patients ranged from 5-20%. In the intention to treat analyses, two studies met the primary endpoint of improvement in disease free survival (CheckMate-274 and AMBASSADOR) while one did not (IMVigor010).3 The pooled results demonstrated significantly improved disease-free survival for patients treated with immune checkpoint inhibition in the intention-to-treat cohorts (HR 0.76, 95% CI 0.65-0.90):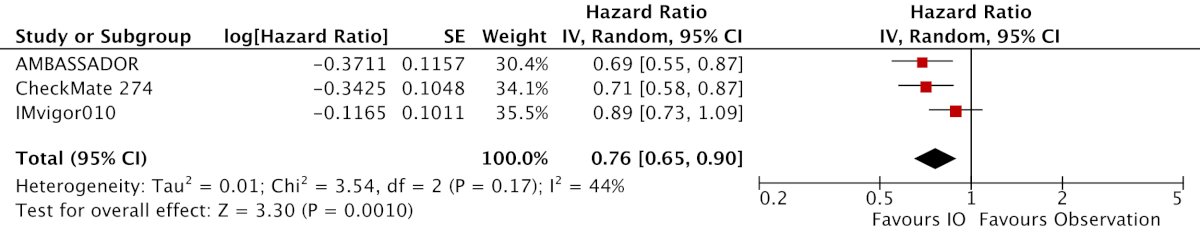
There was considerable clinical and statistical heterogeneity (I2 = 44%) due to differences in inclusion criteria and interventions. Not surprisingly, immune checkpoint inhibitors were associated with greater odds of symptomatic adverse events (OR 1.81, 95% CI 1.31 – 2.49):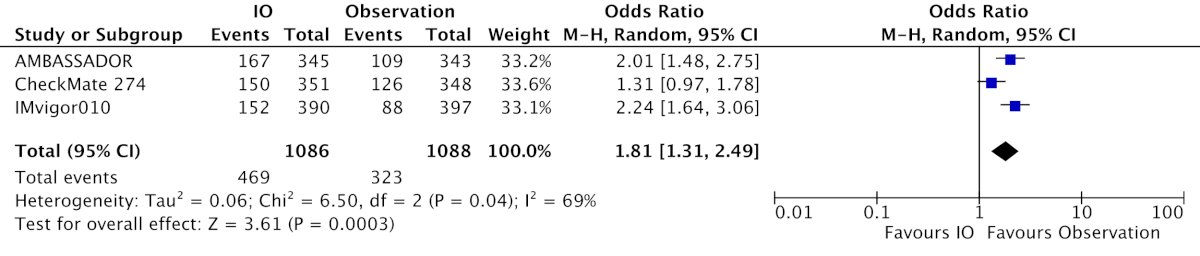
Regarding subgroup analyses, there was significant benefit among patients with negative PD-L1 expression (HR 0.76, 95% CI 0.64-0.90), those who received prior neoadjuvant chemotherapy (HR 0.69, 95% CI 0.52-0.91), and lower tract (HR 0.71, 95% CI 0.55-0.92) but not upper tract disease (HR 1.21, 95% CI 0.87-1.68):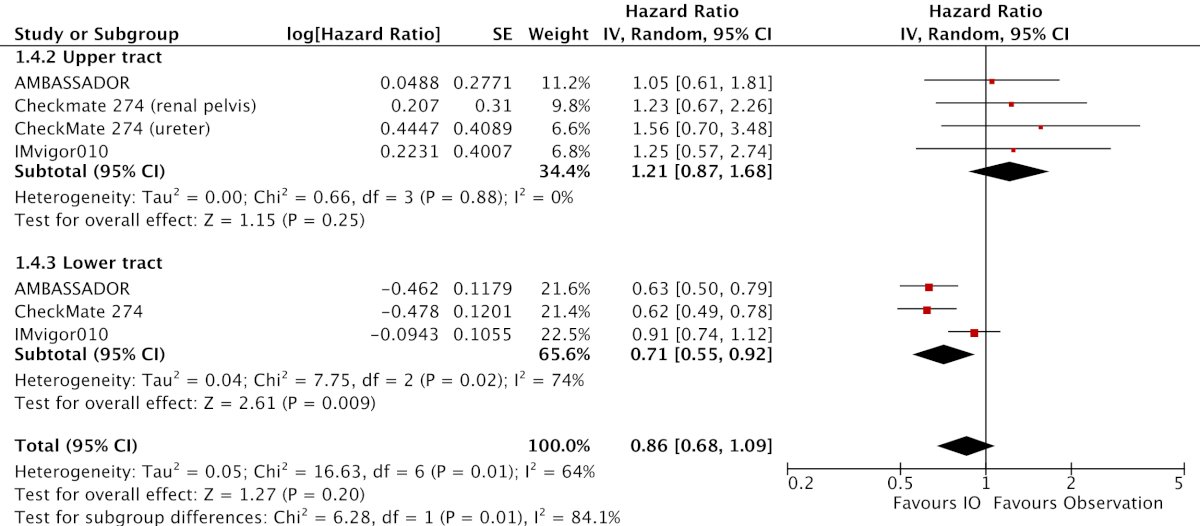
Notably, the subgroup differences for PD-L1 expression (p = 0.93) and previous neoadjuvant chemotherapy (p = 0.13) suggest that immune checkpoint inhibition utilization should not be restricted based on PD-L1 expression or receipt of neoadjuvant chemotherapy.
Dr. Riveros concluded his presentation discussing a systematic review and meta-analysis of randomized clinical trials assessing adjuvant immunotherapy in high-risk muscle invasive urothelial carcinoma by emphasizing that these results support increased adoption of immune checkpoint inhibition following radical resection of muscle invasive urothelial carcinoma.
Presented by: Carlos Riveros, Postdoctoral Research Fellow, Houston Methodist Hospital, Houston, TX
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 South Central American Urological Association (AUA) Annual Meeting, Colorado Springs, CO, Wed, Oct 30 – Sat, Nov 2, 2024.
References:
- Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021 Jun 3;384(22):2102-2114.
- Apolo AB, Ballman KV, Sonpavde G, et al. Adjuvant pembrolizumab versus observation in muscle-invasive urothelial carcinoma. N Engl J Med. 2024 Sep 15 [Epub ahead of print].
- Bellmunt J, Hussain M, Gschwend JE, et al. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): A multicentre, open-label, randomized, phase 3 trial. Lancet Oncol. 2021 Apr;22(4):525-537.


