(UroToday.com) The 2024 South Central AUA annual meeting included a session on kidney cancer, featuring a case discussion on the management of localized renal masses in younger patients and patients with heritable kidney cancer syndromes moderated by Dr. Woodson Smelser hosting urology panelists Drs. Zeynep Gul, Marcelo Bigarella, and Jonathan Heinlen.
Case #1: This is a 35 year old female who presented for counseling due to an “elevated risk of kidney cancer.” Her medical history was significant for a tubal ligation, she was a non-smoker and worked as an accountant. Her family history was notable for leiomyomas and RCC in her mother diagnosed at age 50 (her mother underwent partial nephrectomy and is alive and well), and she noted the presence of “goosebumps” on her skin beginning in her early 30s that have increased in number and breadth.
Dr. Smelser discussed that this is a case of Hereditary Leiomyomatosis and Renal Cell Carcinoma (HLRCC), which is a rare genetic disorder secondary to a mutation in fumarate hydratase, a component of the Krebs Cycle. It follows autosomal dominant inheritance and is characterized by smooth muscle tumors (leiomyomas), with the skin and uterus (fibroids) the most commonly affected sites. Approximately, 10-16% of patients with HLRCC develop kidney cancer.
Dr. Smelser then asked Dr. Heinlen whether he utilizes genetic testing in this population. He noted that if you do not recognize a genetic syndrome in a patient, you likely already have kidney pathology before you do genetic testing. Thus, Dr. Heinlen poses that perhaps we should be doing more biopsies in the initial setting to establish the diagnosis. Regarding how to counsel patients for cascade or family testing, Dr. Gul states that she encourages patients to undergo genetic testing (refers them to a genetic counselor) so it can provide information regarding screening intensity, both for the patient and their family. She also explains to patients the difference between germline and somatic genetic testing, given there can be confusion for these terms for patients.
For counseling patients on the risks of HLRCC tumor compared to other tumors, Dr. Bigarella states that renal mass biopsy in these younger patients is quite helpful if the syndrome is not yet known. If we know that the patient already has HLRCC, he notes that this is a very aggressive type of tumor, with no role for active surveillance and likely the best option would be radical nephrectomy with consideration for retroperitoneal lymph node dissection. However, Dr. Heinlen states that in certain cases it may not be unreasonable to think about a partial rather than radical nephrectomy since we do not have a lot of evidence comparing these two treatment approaches for small, aggressive tumors. For surveillance imaging, Dr. Smelser favors annual MRIs rather than CT scans to decrease the cumulative radiation exposure for the patient.
Case #2: This is a 22 year old male with a history of Von-Hippel-Lindau (VHL) syndrome who presented for an encounter for surveillance. Based on his VHL, he previously had epididymal cystadenomas, in addition to RCC of the right kidney treated with multifocal (6 clear cell RCC tumors, 5 malignant cysts, negative margins on all resections, clamp time was 44 minutes) partial nephrectomy at 19 years of age; his post-p eGFR is 71. Additionally, he is married with an 18 month old son and owns his own construction business. His family history is notable for his father being deceased secondary to RCC when the patient was 2 years of age, and his mother is deceased secondary to metastatic cervical cancer at the age of 42. The patient follows in the heritable cancer clinic at Washington University and has regular screening visits with medical oncology. His wife and son have both had genetic testing and are not VHL affected individuals. A recent MRI was obtained to assess his known burden of renal tumors:
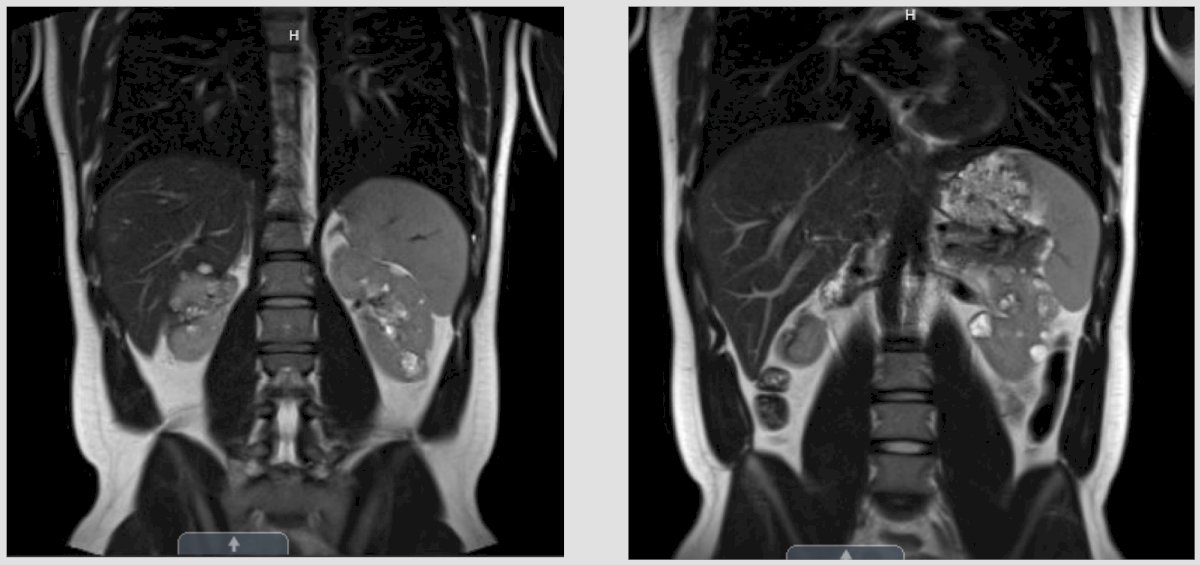
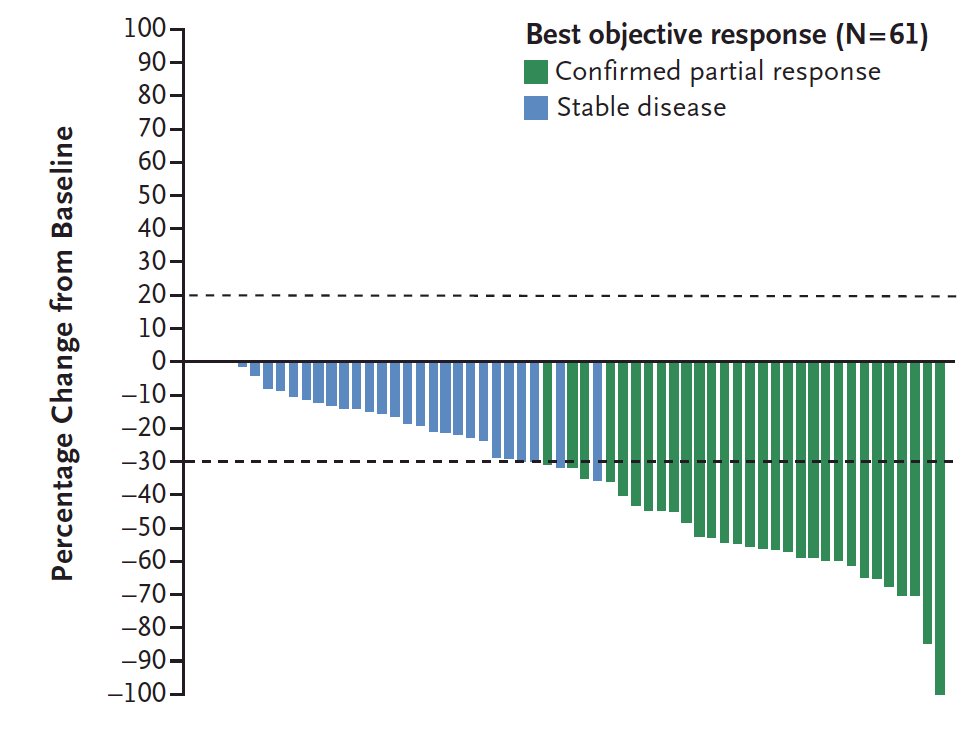
The imaging notes a defect of the right kidney from his prior robotic multiple partial nephrectomy, and on the left kidney, it is evident that there are multiple solid enhancing lesions, with the largest being 3.1 cm in the left upper pole. The first question posed by Dr. Smelser is what are your triggers for intervention for small renal masses in patients with VHL? Dr. Bigarella states that we have the historical cutoff of 3 cm for the largest tumor present in each kidney. However, he notes that we now have belzutifan for VHL patients, which is a HIF-2alpha inhibitor FDA approved in 2021 for VHL patients.1 This approval was based on a phase 2 open-label trial investigated the efficacy and safety of the belzutifan administered orally at a dose of 120 mg daily, in patients with renal cell carcinoma associated with VHL disease. The primary endpoint was objective response (complete or partial response). Over a median follow-up of 21.8 months (range, 20.2 to 30.1 months), the percentage of patients with RCC who had an objective response was 49% (95% CI, 36 to 62). There were no complete responses, but 49% of patients had partial response, 49% had stable disease, and only 3% of patients had progressive disease. Remarkably, a reduction in the sum of all target lesion diameters was observed in 56 patients (92%):
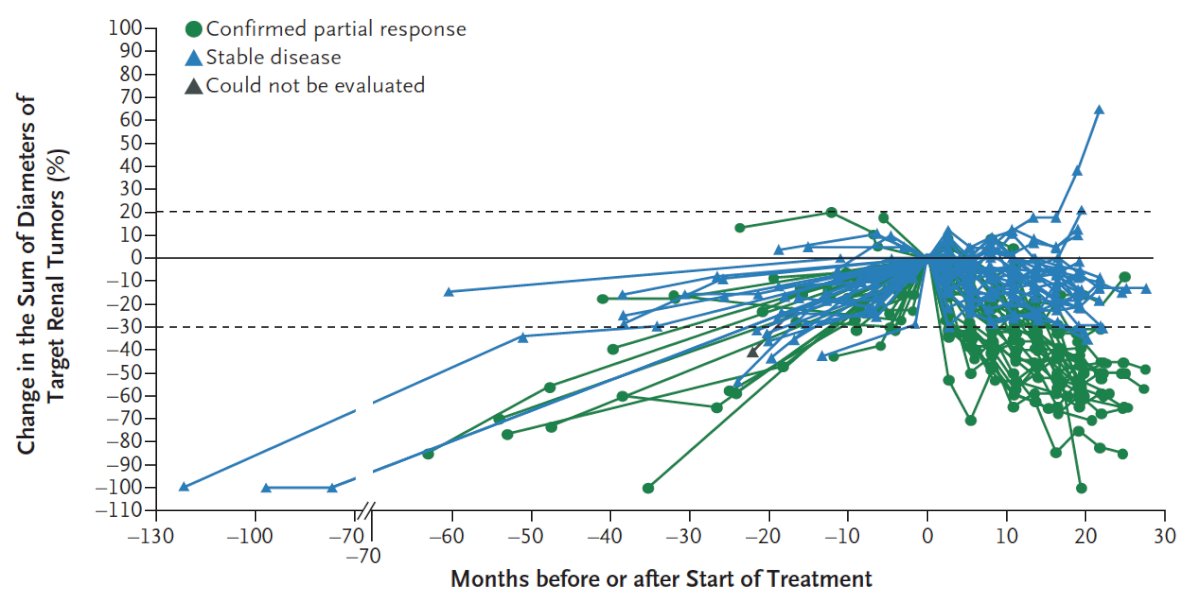
Most patients had growing tumors before treatment, followed by an observed reduction in the sum of the largest tumor diameters after treatment began:
Dr. Heinlen then discussed specific intraoperative considerations for the management of patients with VHL undergoing nephron sparing surgery. He notes that the key for the first surgery on a specific kidney is that you want to resect everything at the time of the first operation. He prefers an open approach for expediency and the ability to provide the kidney cold ischemia, stripping off all of Gerota’s fat before you start resecting tumors. Currently, he does not use mannitol for partial nephrectomies, but in a situation with multiple partial nephrectomies, utilization of mannitol would not be unreasonable. Dr. Smelser does note though that there have been several series published suggesting that robotic multiple partial nephrectomy is safe and feasible for these VHL patients.
Dr. Gul states that there is still some uncertainty with belzutifan, specifically when to start therapy and the duration of therapy. She tends to be more conservative with her use of belzutifan but does refer these patients to medical oncology for a more in depth discussion about the risks and benefits of therapy. The main side effects of belzutifan are anemia and hypoxia, usually within the first month of therapy. Dr. Smelser notes that this patient eventually opted for surgery in which he performed 7 of the 8 robotic partial nephrectomies off clamp after removing Gerotas fascia and fully mobilizing the kidney.
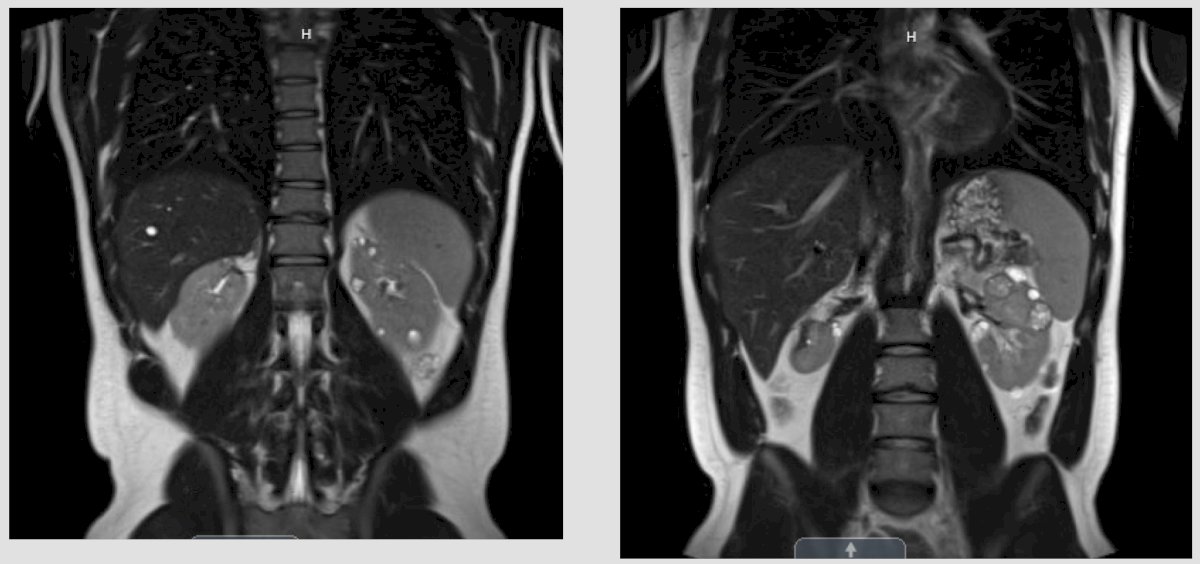
Case #3: This is a 36 year old male who presented to the emergency department with gross hematuria for 3 days. He did not have an appreciable medical or surgical history, he uses cannabis gummies, is engaged, and works in automotive sales. His only medication is lisinopril. His family history included his father with a history of unfavorable intermediate risk prostate cancer treated with a robotic prostatectomy in 2019. The patient’s labs in the emergency department demonstrated a creatinine of 1.3, hemoglobin of 16.7, gross blood on urinalysis, and normal liver function tests. A CT urogram showed a large heterogeneous mass with a renal vein thrombus (not into the IVC):
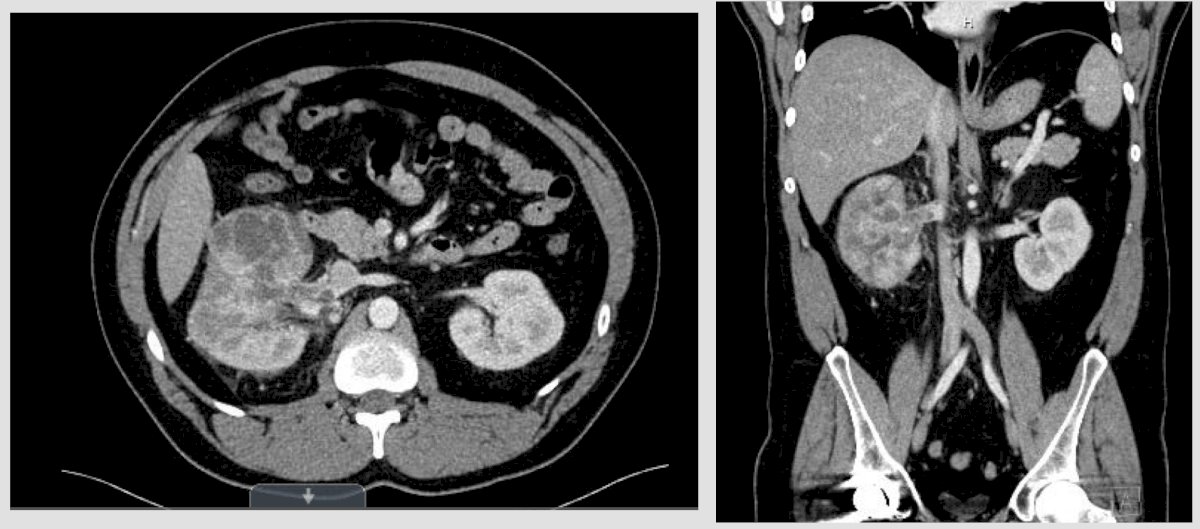
A subsequent MRI confirmed there was no IVC involvement:
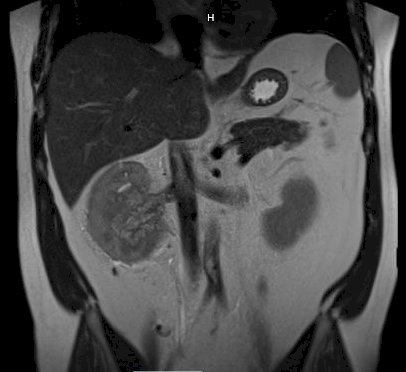
Dr. Heinlen notes that it is important to remember that the patient had gross hematuria, thus he needs a cystoscopy to rule out a bladder lesion, and potentially even a ureteroscopy to ensure this is not a large renal pelvis tumor, which would change the management. Surgically, he notes that this appears to be resectable, and given that there is no IVC involvement, he would perform this robotically. Dr. Bigarella states that for right sided renal vein thrombus patients he prefers an open approach because of the short right renal vein, but if this were a left sided tumor, he would perform this robotically given the extra left renal vein length. This patient underwent a robotic right radical nephrectomy with retroperitoneal lymph node dissection with Dr. Smelser, pathology was noted to be pT3a clear cell RCC, without sarcomatoid features. He also had genetic testing completed, which was negative. Regarding the possibility of adjuvant therapy, Dr. Gul notes that given his aggressive disease at a young age, it would be reasonable to offer this patient adjuvant therapy. She sends all of her patients to medical oncology for an opinion regarding adjuvant therapy, emphasizing that she has a lot of older patients that do not opt for treatment given their age and decreased long-term risk of recurrence.
Moderator: Woodson Smelser, MD, Washington University, St. Louis, MO
Panelists:
- Zeynep Gul, MD, Washington University, St. Louis, MO
- Marcelo Bigarella, MD, University of Arkansas, Little Rock, AR
- Jonathan Heinlen, MD, University of Oklahoma, Oklahoma City, OK
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 South Central American Urological Association (AUA) Annual Meeting, Colorado Springs, CO, Wed, Oct 30 – Sat, Nov 2, 2024.
References:


