(UroToday.com) The 2024 South Central AUA annual meeting included a session on bladder cancer, featuring a presentation by Dr. Yair Lotan discussing 36-month follow-up from a phase 3 trial assessing the efficacy of intravesical nadofaragene firadenovec for patients with BCG-unresponsive non muscle invasive bladder cancer (NMIBC).
Local bladder-preserving treatment options are needed for patients with BCG-unresponsive NMIBC. Nadofaragene firadenovec-vncg, a non-replicating adenovirus vector-based gene therapy, is approved by the US FDA for patients with BCG-unresponsive NMIBC with carcinoma in situ (CIS) with/without papillary tumors (±Ta/T1). The primary endpoint of the open-label, multicenter, phase 3 study of nadofaragene firadenovec was met, as 53.4% (95% CI 43.3, 63.3) of patients with CIS ± Ta/T1 achieved complete response at 3 months.1 At the 2024 South Central AUA annual meeting, Dr. Lotan and colleagues presented 36-month follow-up results of this trial.
Patients with BCG-unresponsive NMIBC (n = 157) were enrolled, including 107 and 50 patients were in the CIS ± Ta/T and Ta/T1 without CIS (papillary disease) cohorts, respectively. Efficacy analysis included 103 and 48 patients in the CIS and papillary disease cohorts, respectively, who met the protocol definition of BCG-unresponsive NMIBC. Patients received nadofaragene firadenovec once every 3 months with cystoscopy and cytology assessments. Biopsies were taken at 12 months and patients who remained high-grade recurrence-free were offered continued treatment at the investigator’s discretion: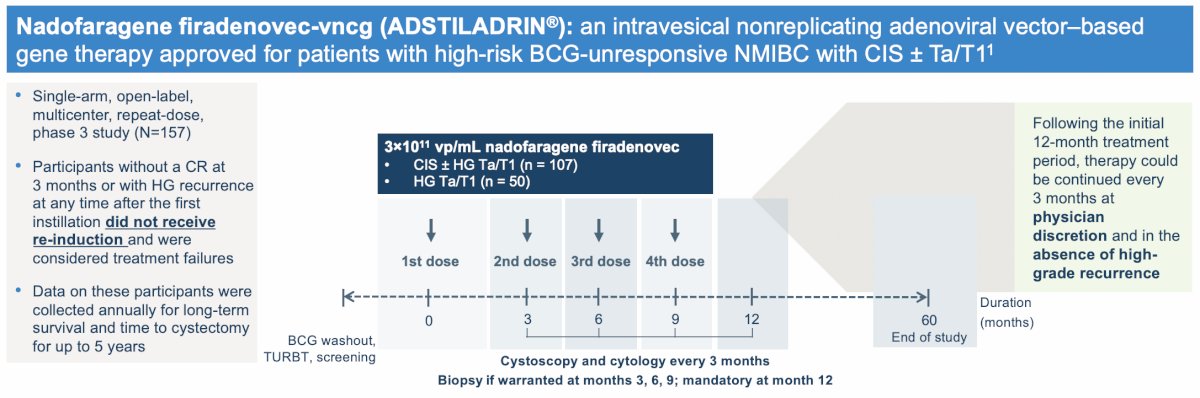
Notably, participants without a complete response or with high grade recurrence at any time after the first instillation did not receive re-induction and were withdrawn from further treatment. However, data on these patients were still collected annually for long-term survival and time to cystectomy for up to 5 years.
All patients completed the 36-month visit or discontinued by September 9, 2021. The mean duration of follow-up was 42.6 (SD 12.2) months. In terms of the primary endpoint, 53.4% of participants (55 out of 103) in the CIS cohort experienced a complete response by month 3 after the first dose of nadofaragene firadenovec. By month 36, 13.6% (14 of 103) in the CIS cohort remained free from high-grade recurrence, and of the 55 participants who were high-grade recurrence free by month 3, 25.5% remained high-grade recurrence free by month 36: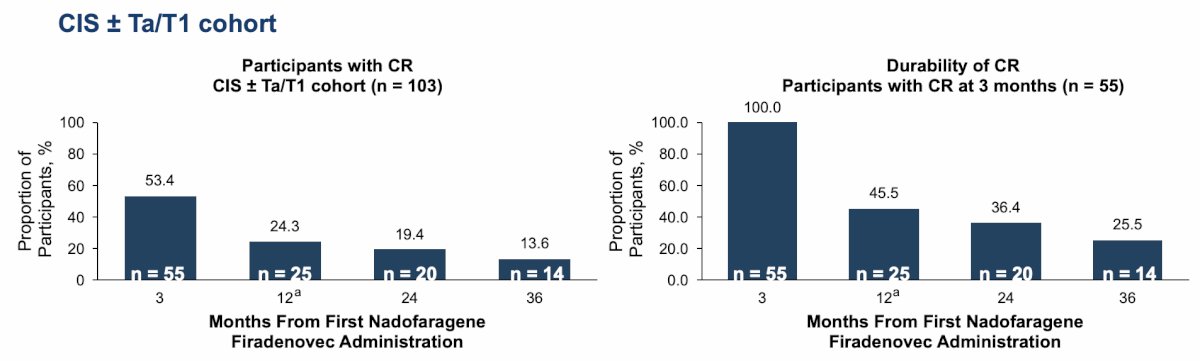
72.9% of participants (35 out of 48) in the high-grade Ta/T1 cohort were high-grade recurrence free by month 3 after the first dose of nadofaragene firadenovec. By month 36, 22.9% (11 of 48) remained free from high-grade recurrence, and of the 35 participants who were high-grade recurrence free by month 3, 31.4% remained high-grade recurrence free by month 36: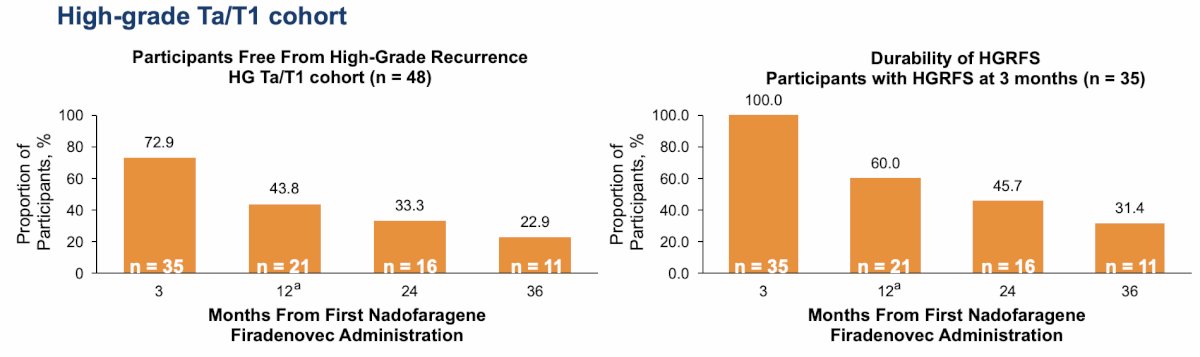
The median duration of complete response was 9.7 months (95% CI 9.2 – 24.0), and the probability of duration of complete response for at least 36 months was 34.2% (95% CI 21.6 – 47.1%):

In the CIS cohort, the median duration of high-grade recurrence-free survival was 6 months (95% CI 3.4-8.3), while in the high-grade Ta/T1 cohort, the median duration of high-grade recurrence-free survival was 12.4 months (95% CI 6.7-23.0):
Nadofaragene firadenovec is a potential bladder-sparing intravesical therapy, as the overall cystectomy-free survival rate through 36 months was 56.9%. Cystectomy-free survival was 53.8% in the CIS cohort and 63.6% in the Ta/T1 cohort, and overall survival was 90.7%, with no treatment-related deaths: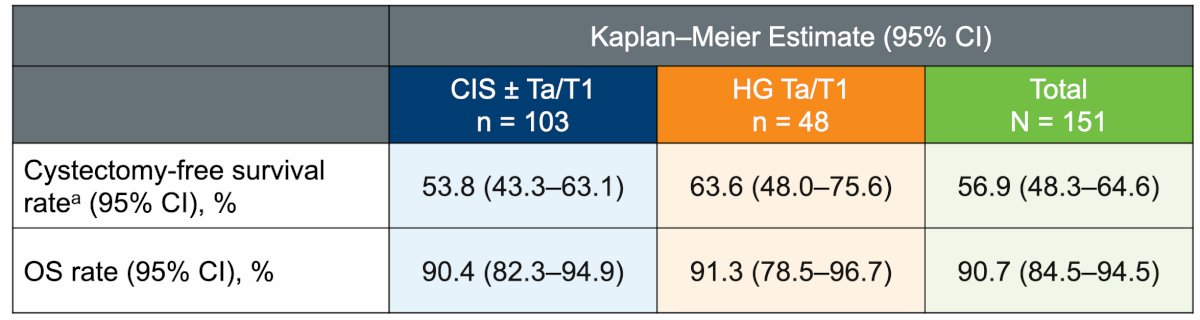
There were few discontinuations due to adverse events, with three participants discontinuing due to an adverse event. There were no new serious adverse events or safety signals observed through 36 months of follow-up.
Dr. Lotan concluded his presentation discussing 36-month follow-up from a phase 3 trial assessing the efficacy of intravesical nadofaragene firadenovec for patients with BCG-unresponsive NMIBC with the following take-home points:
- 53.4% of participants with CIS achieved a complete response at 3 months, with no re-induction among those that not achieve a complete response by 3 months
- 25.5% of participants with CIS with an initial complete response by 3 months remained high-grade recurrence free through 36 months, with a median duration of complete response of 9.7 months
- 31% of participants with Ta/T1 who were high-grade recurrence free by 3 months remained high-grade recurrence free through 36 months
- More than half of participants achieved cystectomy-free survival by 36 months: 53.8% in the CIS cohort and 63.6% in the Ta/T1 cohort
- No new safety signals were identified through 36 months of follow-up
- Nadofaragene firadenovec is a well-tolerated, safe, and effective intravesical therapy with a convenient dosing schedule that provides a novel treatment option for patients with CIS ± Ta/T1 and HG Ta/T1 who are unwilling or unable to undergo radical cystectomy
Presented by: Yair Lotan, MD, Urologist, UT Southwestern University, Dallas, TX
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 South Central American Urological Association (AUA) Annual Meeting, Colorado Springs, CO, Wed, Oct 30 – Sat, Nov 2, 2024.
References:
Video Related Content: 36-Month Efficacy of Nadofaragene in BCG-Unresponsive Non-Muscle-Invasive Bladder Cancer - Yair Lotan

