(UroToday.com) The 2024 South Central AUA annual meeting included the Sushil Lacy manuscript competition, featuring a presentation by Dr. Amanda Myers discussing the elucidation of response rates to additional BCG and implications for clinical trial design. Intravesical BCG remains the most effective treatment for high-grade nonmuscle invasive bladder cancer (NMIBC), so much so that clinical trials are designed around ‘categories’ of recurrences after BCG.
“BCG-unresponsive” is strictly defined by the FDA, whereas “BCG-exposed” includes BCG failures that do not meet BCG-unresponsive criteria. Clinical trials are often powered based on historical response rates in BCG naïve patients, leading to underpowered studies. To help inform the field, Dr. Myers and colleagues reported the response rate to additional BCG in patients with BCG-exposed and BCG-unresponsive NMIBC.
This study included an IRB-approved review of consecutive patients diagnosed with NMIBC between January 2000 and September 2021. The investigators analyzed the outcomes of patients who received additional BCG as primary therapy and who met published criteria for BCG-exposed and BCG-unresponsive definitions. The primary outcome was high-grade disease-free survival. The Kaplan-Meier method was used to estimate disease-free survival, cystectomy-free rate, progression to muscle-invasive or metastatic-free survival, and overall survival.
From 857 patients treated with BCG at MD Anderson Cancer Center, they identified 88 patients with high-grade recurrences treated with additional BCG: BCG-exposed n = 52 and BCG-unresponsive n = 36. Tumor characteristics and BCG response are as follows: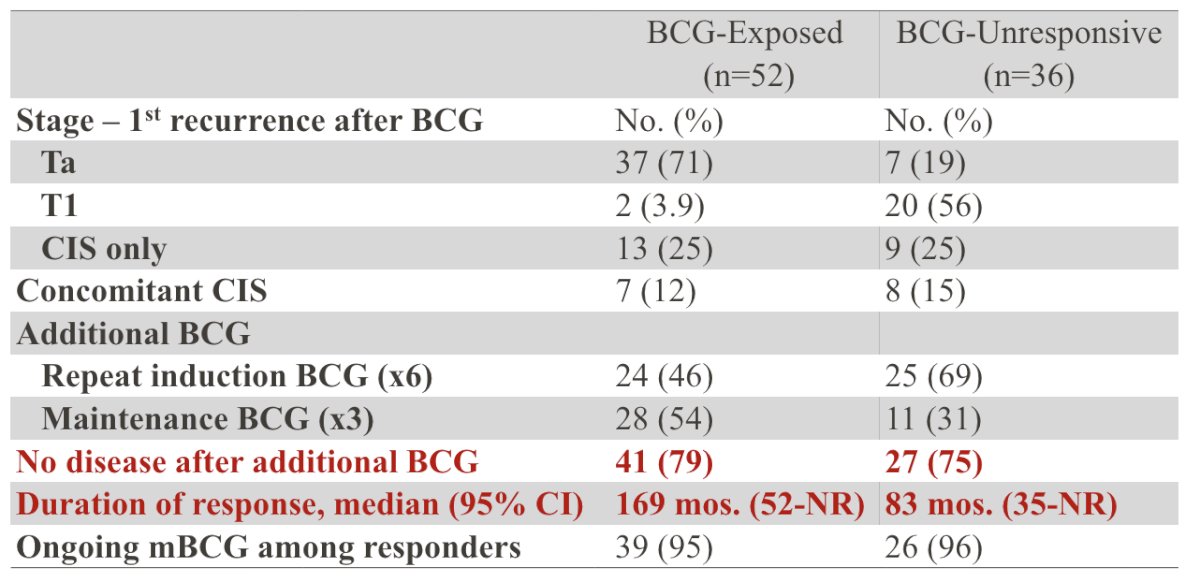
The median follow-up was 4.2 years (IQR 2.5-8.5). For BCG-exposed patients, treatment with additional BCG resulted in complete response in 41 (79%) patients with a median duration of response among responders of 169 months (95% CI 52 months - NR). For BCG-unresponsive patients, 27 (75%) patients remained disease-free after additional BCG, with a median duration of response of 83 months (95% CI 35 - NR). At the two-year landmark analysis, disease-free survival for BCG-exposed was 63% (95% CI 51-78), and for BCG-unresponsive was 63% (95% CI 48-81):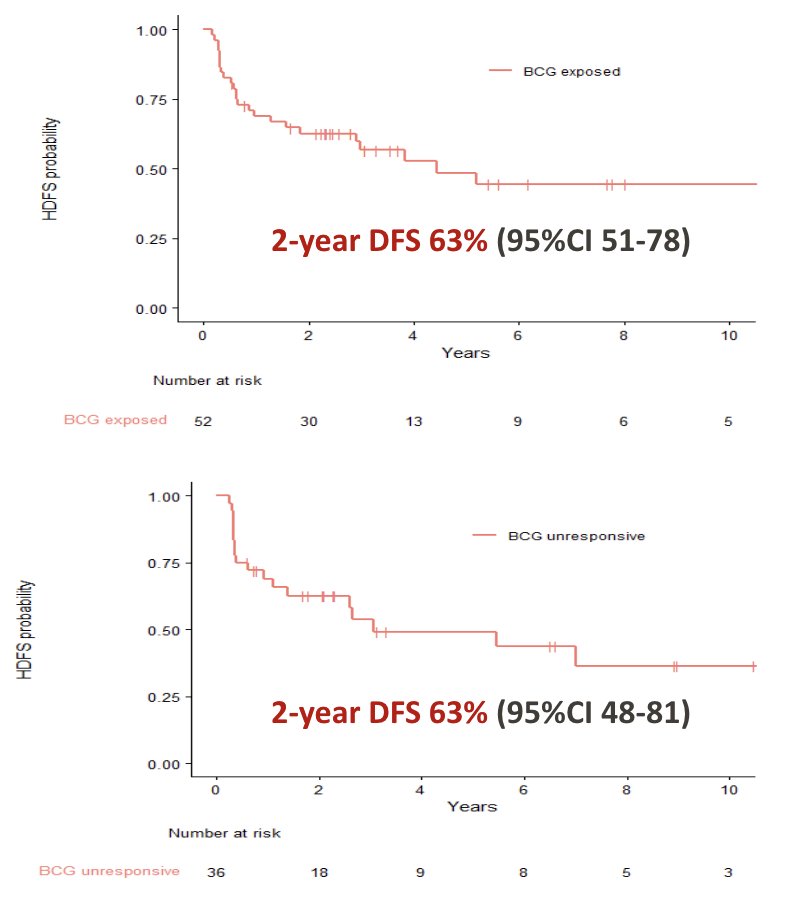
At the 2-year landmark analysis, for BCG exposed patients, progression-free survival was 90% (95% CI 82-99), and for BCG unresponsive patients was 86% (95% CI 75-98):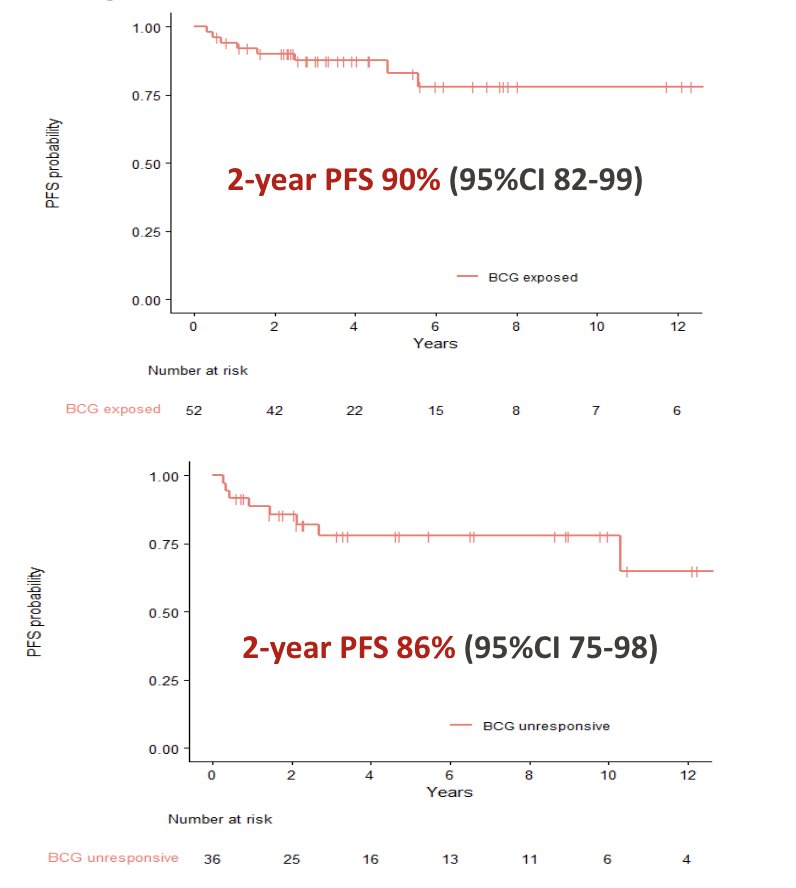
For BCG exposed patients, the 2-year cystectomy-free rate was 82% (95% CI 72-93) and overall survival rate was 96% (95% CI 91-100); for BCG unresponsive patients, the 2-year overall survival rate was 91% (95% CI 83-100).
Dr. Myers concluded her presentation discussing the elucidation of response rates to additional BCG and implications for clinical trial design with the following take-home points:
- This study provides reference data for response rates to additional BCG for patients with NMIBC
- This data serves as a comparator for nonrandomized studies and can inform power calculations for randomized studies
- This is the first study to evaluate the response to additional BCG using the BCG-exposed definition
- Given the high response rates to additional BCG in the BCG-exposed groups, it is essential that clinical trials in this population compare against BCG as a control arm.
Presented by: Amanda Myers, MD, University of Texas MD Anderson Cancer Center, Houston, TX
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 South Central American Urological Association (AUA) Annual Meeting, Colorado Springs, CO, Wed, Oct 30 – Sat, Nov 2, 2024.


