Dr. Guru Sonpavde (Dana-Farber Cancer Institute) gave the first talk of the session, focusing on germline evaluation for UC. Mounting evidence from genetic studies demonstrates an impact of both heritable gene variants and family history of UC on the development of the disease.
After highlighting studies demonstrating familial risk and heritability for UC, including a Nordic study in twins which showed significant heritability of up to 33%,1 Dr. Sonpavde tackled the topic of universal versus guideline-based germline genetic testing. In a series of 1040 patients with advanced cancer, 101 of 182 patients with clinically actionable inherited mutations detected by tumour-normal sequencing would not have been detected by guideline-based testing.2 Furthermore, in a multicentre cohort study analyzing a gene panel of 2984 unselected patients with solid tumours, 1 in 8 patients had germline alterations across all tumours (including 106 bladder cancer patients), half of which would not be detected had guideline-based testing been used.3 Universal germline genetic testing may therefore provide a superior approach and momentum for this is building.
In the second presentation of the session, Dr. Kent Mouw (Dana-Farber Cancer Institute) discussed germline and somatic DNA repair alterations in UC. Germline mismatch repair (MMR) mutations are seen in Lynch syndrome but sporadic (somatic) MMR deficiency may also occur, carrying important implications for UC diagnosis, treatment, and surveillance. Dr. Mouw also discussed the role of ERCC2, a member of the nucleotide excision repair (NER) pathway, which repairs bulky intra-strand DNA adducts. Somatic ERCC2 mutations are present in 10% of bladder tumours and are associated with improved response to cisplatin-based chemotherapy.4 Additionally, homologous recombination (HR) deficiency may also be present in a small subset of UC although identification of these cases is challenging. HR is clinically relevant because HR-deficient tumours are more sensitive to poly (ADP-ribose) polymerase inhibitors (PARPi). To this end, Dr. Mouw highlighted the ATLAS and BISCAY trials, both of which are evaluating PARPi in patients with UC. The phase II ATLAS trial enrolled 97 patients with previously treated advanced UC.5 Patients received rucaparib and next-generation sequencing was used to assess mutation status and loss of heterozygosity (LOH). Although the ORR was 0%, a reduction in tumour size was observed in a number of patients. Currently, the role of PARPi in UC is unclear but ongoing trials such as BAYOU (NCT03459846), NEODUVARIB (NCT03534492), and NCI (NCT03375307) are anticipated to shed further light on their role in this disease.
In the final talk of the session, Dr. Faltas (Weill Cornell Medicine) described his work using whole-exome sequencing (WES) to identify putative deleterious germline variants (pDGVs) in patients with UC. Dr. Faltas and his team performed WES of germline samples in 157 tumors from 80 advanced UC patients at Weill Cornell Medicine.6 A stepwise computational framework was developed to distinguish pDGVs from a large number of background germline variants in each UC patient. Putative deleterious germline variants were identified in 56% of patients with UC and the majority of these were either stop gain or frameshift mutations in tumour suppressor genes. To validate these findings, the team performed a similar analysis using raw data from The Cancer Genome Atlas (TCGA) bladder cancer cohort and identified pDGVs in 48% of this cohort. These pDGVs commonly affected DNA repair genes including POLQ, FANCA, and BRCA2. Furthermore, pDGVs were significantly enriched in patients with UC compared to non-cancer subjects in both TGCA and Weill-Cornell cohorts.
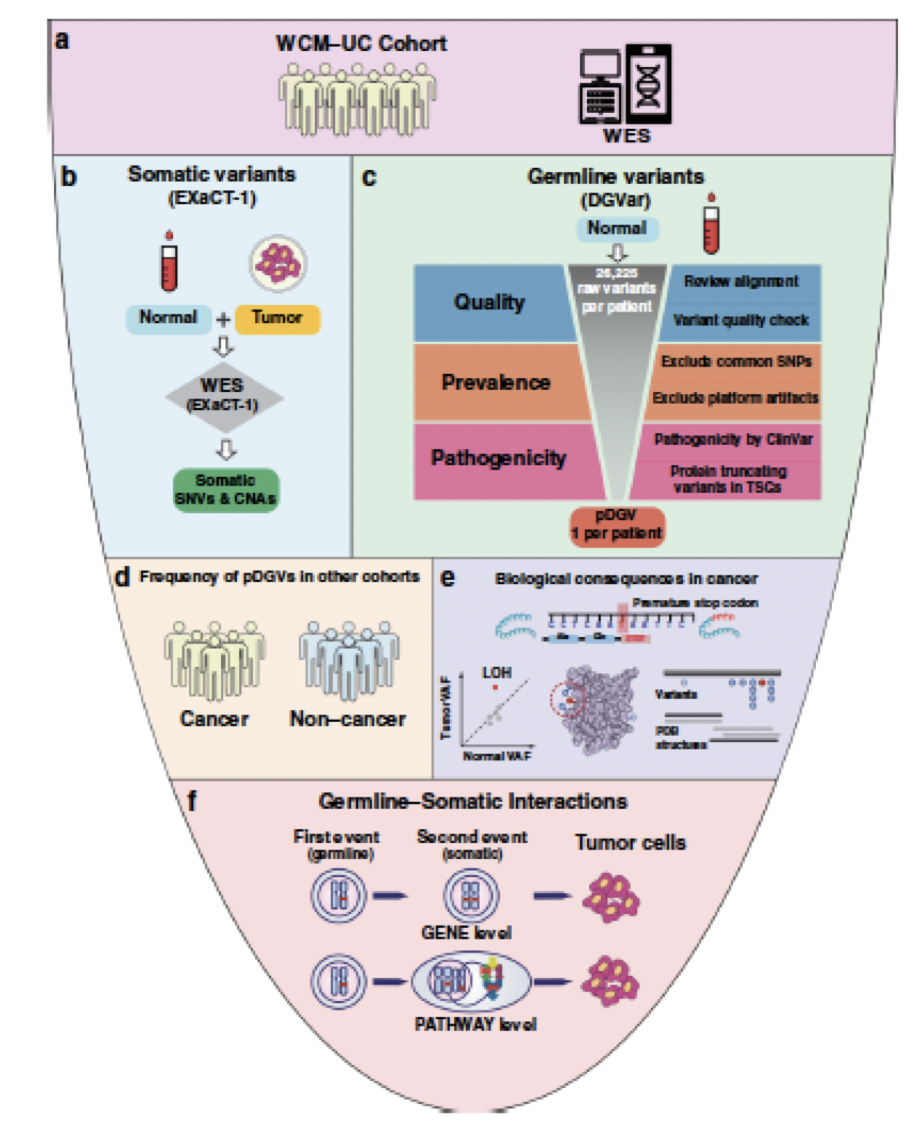
The team then turned their attention to understanding the nature and biological consequences of pDGVs in tumours, and found that 53% of pDGVs showed evidence of somatic LOH. Furthermore, a significant deepening LOH was observed in metastatic tumours compared to the primary tumours, suggesting that the evolutionary pressure of these pDGVs drives progressive LOH in metastatic UC.
Dr. Faltas ended his talk with the following summary points:
- Germline mutations affecting DNA damage repair genes are common in patients with UC.
- Progressive LOH in serial tumour samples suggests a critical role for pDGVs in tumour progression.
- Significant intra-patient heterogeneity in germline somatic variant interactions results in divergent biological processes in the primary and metastatic tumours.
- Germline variation adds another layer of complexity to clinical genomics that needs to be considered for precision medicine strategies.
Presented by: Bishoy Faltas, MD, Weill Cornell Medicine; Guru Sonpavde, MD, Dana-Farber Cancer Institute; & Kent Mouw, MD, PhD, Dana-Farber Cancer Institute
Written by: Niyati Lobo, MD, The Urology Foundation Fulbright Scholar, Twitter: @niyatilobo, with Ashish Kamat, MD, MBBS, President, International Bladder Cancer Group (IBCG), Professor of Urology & Cancer Research, MD Anderson Cancer Center, Houston, Texas, Twitter: @UroDocAsh at the 7th Annual Albert Institute for Bladder Cancer Care and Research (AIBCCR) Symposium, Sept 16, 2021- Sep 18, 2021.
References:
- Mucci LA, Hjelmborg JB, Harris JR et al. Familial risk and heritability of cancer among twins in Nordic countries. JAMA 2016; 315: 68-76
- Mandelker D, Zhang L, Kemel Y et al. Mutation detection in patients with advanced cancer by universal sequencing of cancer-related genes in tumour and normal DNA vs guideline-based germline testing. JAMA 2017; 318: 825-835
- Sammader NJ, Riegert-Johnson D, Boardman L et al. Comparison of universal genetic testing vs guideline-directed targeted testing for patietns with hereditary cancer syndrome. JAMA Oncol. 2021; 7: 230- 237
- Li Q, Damish AW, Frazier Z, Liu D, Reznichenko E, Kamburov A, et al. ERCC2 helicase domain mutations confer nucleotide excision repair deficiency and drive cisplatin sensitivity in muscle-invasive bladder cancer. Clin Cancer Res. 2019;25:977–88.
- Grivas P, Loriot Y, Morales-Barrera R et al. Efficacy and safety of rucaparib in previously treated, locally advanced or metastatic urothelial carcinoma from a phase 2, open-label trial (ATLAS). BMC Cancer. 2021; 21: 593
- Vosoughi A, Zhang T, Shohdy KS et al. Common germine-somatic variant interactions in advanced urothelial cancer. Nat Commun. 2020: 11: 619


