Dr. Hofman began by describing the TheraP trial. This ANZUP/PCFA sponsored trial began in 2016. It represents a world-first study, being a Phase III randomized controlled trial of a direct targeted radioligand. In particular, TheraP enrolled patients with metastatic castration-resistant prostate cancer (mCRPC) who had previously received docetaxel and were eligible to receive cabazitaxel. Patients were required to have progressive disease with a rising prostate-specific antigen (PSA) with an absolute PSA of 20 ng/mL or higher.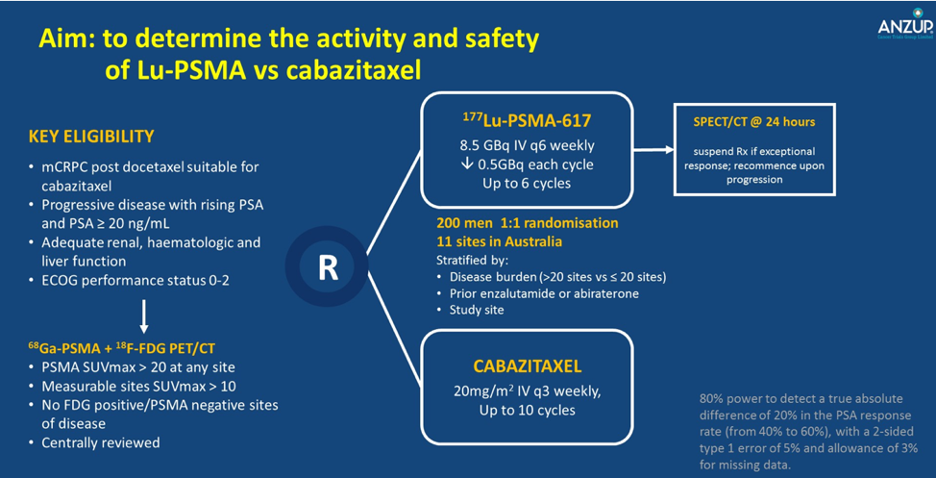
All patients underwent both 68Ga-PSMA-PET/CT and 18F-FDG-PET/CT prior to randomization. To be eligible for inclusion, patients must have had a high avidity lesion on PSMA PET/CT (SUV max >20 at any site) with measurable disease with SUV max of 10 or greater. Further, there could not be sites of disease which were FDG positive but PSMA negative.
Among 200 men at 11 sites in Australia who were eligible, randomization was performed in a 1:1 fashion to 177Lu-PSMA-617 or cabazitaxel. Randomization was stratified according to disease burden, prior use of enzalutamide or abiraterone, and study site.
The primary study outcome was PSA response and these data were initially reported at the 2020 American Society of Clinical Oncology (ASCO) Virtual Congress. PSA response was operationalized looking at a response of at least 50% from baseline. Compared to those receiving cabazitaxel (37%, 95% confidence interval 27 to 46%), responses were significant higher among those who received Lu-PSMA (66%, 95% confidence interval 56 to 75%) with an absolute difference of 29% (95% confidence interval 16 to 42%, p<0.0001).
Further, patients who received Lu-PSMA experienced lower rates of toxicity.
Moving forward, the next analysis of this cohort is pending and expected to be reported at ASCO-GU 2021 examining progression-free survival (both PSA-based and radiographic) and quality of life outcomes. A manuscript is also anticipated in early 2021.
Dr. Hofman highlighted that this is the first reported trial using Lu-PSMA as a theranostic. The ongoing VISION trial shares some similarities but many differences. While also assessing PSMA-targeted radioligands, VISION is an industry-sponsored trial with a much larger sample size (800 patients). Dr. Hofman highlighted two key differences:
#1. The manner in which the agent is produced: in VISION, a central production model is used with the agent subsequently shipped ready for clinical use. In contrast, in TheraP, lutetium is made on site. This allows some flexibility but required standardization and quality control in order to undertake this trial. Moving forward, this approach is expected to be much lower cost than centralized processing.
#2. Patient selection: As mentioned above, in order to be included in TheraP, patients could not have sites of disease which were FDG positive but PSMA negative. This requirement led to the exclusion of approximately 30% of otherwise eligible men. In contrast, the VISION trial didn’t have this requirement. Dr. Hofman suggested that this narrowing of the selection criteria will likely enrich TheraP for patients who are likely to respond. As a result, we should expect that the magnitude of benefit will be larger in the TheraP trial.
Dr. Peter Grimison then discussed questions of how to transition Lu-PSMA to clinical use, focusing on pragmatic issues. From the perspective of a pharmaceutical benefit approach, he emphasized that there are numerous steps required including (1) demonstrating activity and efficacy, (2) demonstrating safety, and (3) demonstrating cost-effectiveness prior to funding decisions. In the first step, the therapeutic goods administration can consider efficacy and safety in isolation, without comparison to other agents. Approval at this step makes the agent available but not funded. Secondarily, the pharmaceutical benefits advisory committee makes recommendations to the government regarding funding. This process differs from in the US in which agents are approved following demonstration of efficacy, without contextualizing in health economic modeling.
Dr. Emmett then asked where we are heading next with Lu-PSMA. Dr. Hofman suggested that there are two major issues: first, how to get access to Lu-PSMA in patients meeting TheraP inclusion criteria and, two, to consider how else to use Lu-PSMA, including potentially earlier in the disease process and in combination with other approved agents in this disease space.
Presented by: Louise Emmett, MBBS, FRACP, FAANMS, MD, Director of Theranostics and Nuclear Medicine, St. Vincent's Hospital, Clinical Research Leader, Clinical Prostate Cancer Research Group, Garvan Institute of Medical Research, Conjoint Professor, the University of New South Wales (UNSW), Sydney, Australia
Michael Hofman, MBBS (Hons), FRACP, FAANMS, Professor Michael Hofman is a nuclear medicine physician and physician-scientist. Peter MacCallum Cancer Center, Victoria, Australia
Written by: Christopher J.D. Wallis, MD, PhD, Urologic Oncology Fellow, Vanderbilt University Medical Center, Nashville, Tennessee, Twitter: @WallisCJD, during the 2020 Australian and New Zealand Urogenital and Prostate Cancer Trials Group (ANZUP) Mini Annual Scientific Meeting (ASM), November 29 - 30, 2020
Related Content:
TheraP: 177Lu-PSMA617 Theranostic vs Cabazitaxel in Progressive Metastatic Castration Resistant Prostate Cancer (mCRPC) - Michael Hofman & Ian Davis
The VISION Trial: Radionuclide Therapy Plus Standard Therapy for Metastatic Castration Resistant Prostate Cancer - Oliver Sartor and Michael Morris


