The standards for trial conduct for nmCRPC trials were established from the phase III trial of denosumab versus placebo.1 This trial had 1,432 men with nmCRPC randomized to denosumab or placebo, and eligible patients had a high risk of metastatic disease: PSA >8ng/mL, and/or PSA doubling time <= 10 months. The primary endpoint was bone metastasis-free survival (BMFS), and the trial was powered to a hazard ratio for denosumab versus placebo of 0.8. The results of this trial represented a significant improvement in BMFS for denosumab, but a median 4.2-month difference was too low for approval by the ODAC based on the adverse event profile. Furthermore, there was no overall survival (OS) benefit for denosumab (HR 1.1, 95% CI 0.85-1.20).
Trial designs aim to balance a need for treatment for largely asymptomatic patients, ensuring a number of events to show efficacy relative to safety. PSA doubling time is the most widely used prognostic factor to determine risk, refined from outcomes of patients treated in the placebo arm of phase 3 placebo-controlled denosumab registration trial. Metastasis free survival (MFS) is the primary endpoint—the time from randomization to the first detection of disease in bone or death from any cause. The relative risk for BMFS in the placebo arm of the denosumab phase 3 trial shows a major inflection point at a PSA doubling time of 8 months:
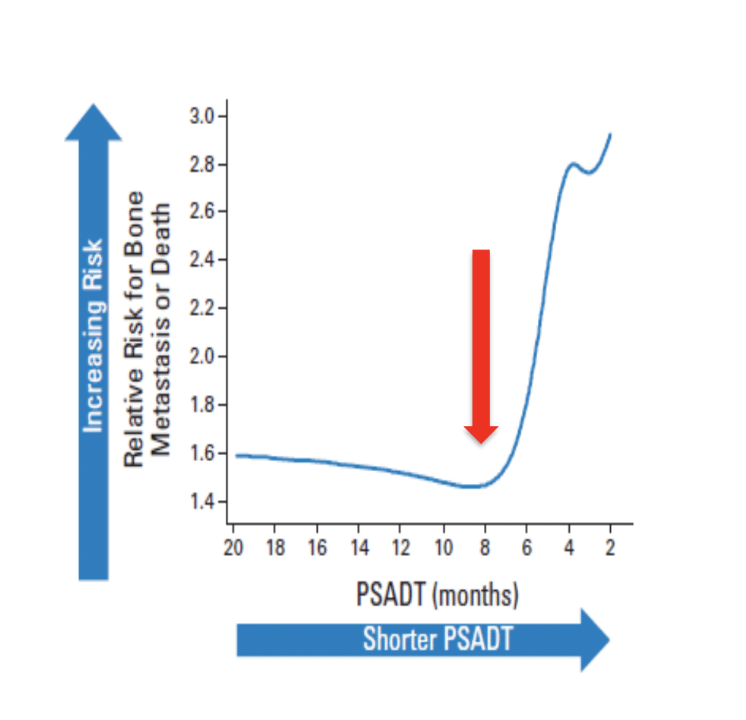
Based on these results, most contemporary trials use a PSA doubling time of <8 or <10 months for enrollment.
Therapeutic options in this disease space have increased tremendously in the past few years, starting with the bone targeting agents (bisphosphonates, denosumab, etc), endothelin receptor A antagonists (atrasentan, zibotentan), miscellaneous agents (bevacizumab, cilengitide,somatostatin, octreotide), and more recently the next generation hormonal agents (enzalutamide, apalutamide, darolutamide). The details of these AR targeted studies will be discussed in more detail in subsequent talks in this session, but Dr. Scher notes that he is impressed that these trials were able to be conducted, given that it is hard to keep patients with rising PSA’s on study until the primary metastatic endpoint is met. Now, as the drugs approved for the nmCRPC and mCRPC state are studied in the non-castrate states, progressing and relapsing CRPCs will be more diverse biologically.
As has been discussed at length at APCCC 2019, improved imaging has resulted in a stage migration, as more specific and sensitive imaging modalities (ie. PSMA PET-CT) decreases the nmCRPC space. Indeed, prospective evaluation in biochemical recurrence notes that the higher the PSA, the higher the positivity rate of finding micrometastatic disease sites.
According to Dr. Scher, imaging biomarker development needs to focus on the context of use:
- Context–the management/treatment decisions that the biomarker result will be used to inform
- Method (analytic)validation–the process of assessing the device and its measurement performance characteristics, and determining the range of conditions under which the assay will give reproducible and accurate data
- Clinical validation–the evidentiary process of linking a biomarker with biological processes and clinical endpoints
- Clinical utility–showing that use of the test to inform management improves patient outcomes relative to non-use of the test
Dr. Scher concluded that nmCRPC is a moving target:
- The FDA now recognizes that the development of metastatic disease is an objective and clinically relevant measure
- The range of options has increased significantly but future cohorts presenting for treatment will be more biologically diverse
- PSMA PET is changing practice but nmCRPC still exists – further development needs more focus on pre-specified management decisions with regulatory/coverage considerations
- Optimal management should include shared decision making, balancing patient needs, and drug safety
Presented by: Howard I. Scher, MD, Medical Oncologist, Co-Chair, Center for Mechanism-Based Therapy; Head of the Biomarker Development Initiative; D. Wayne Calloway Chair in Urologic Oncology, Memorial Sloan Kettering Cancer Center, New York, NY
Written by: Zachary Klaassen, MD,MSc–Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the 2019 Advanced Prostate Cancer Consensus Conference (APCCC) #APCCC19, Aug 29 - 31, 2019 in Basel, Switzerland
References:
- Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: Results of a phase 3, randomized, placebo-controlled trial. Lancet2012 Jan7;379(9810):39-46. Speaker: Howard I. Scher, Memorial Sloan Kettering Cancer Center, New York, NY
Further Related Content: The Definition and Overview of Treatment Options in nmCRPC Presentation


