The basis for antiresorptive therapy to reduce SRE risk in men with bone-metastatic CRPC dates back nearly two decades now to Dr. Fred Saad’s seminal work in 2002. The phase III zoledronic acid 039 trial showed that among men with mCRPC a greater proportion of patients who received placebo had skeletal-related events than those who received zoledronic acid at 4 mg (44.2% versus 33.2% p =0.021) or those who received zoledronic acid at 8/4 mg (38.5% p = 0.222)1. Furthermore, the median time to the first skeletal-related event was 321 days for patients who received placebo, was not reached for patients who received zoledronic acid at 4 mg (p = 0.011 versus placebo), and was 363 days for those who received zoledronic acid at 8/4 mg (p = 0.491 versus placebo). Pain and analgesic scores increased more in patients who received placebo than in patients who received zoledronic acid, but there were no differences in disease progression, performance status, or quality-of-life scores among the groups. This clinical trial, published nearly 20 years ago, was crucial in bringing bone health and bone-targeted therapy to the forefront of bone metastasis treatment.
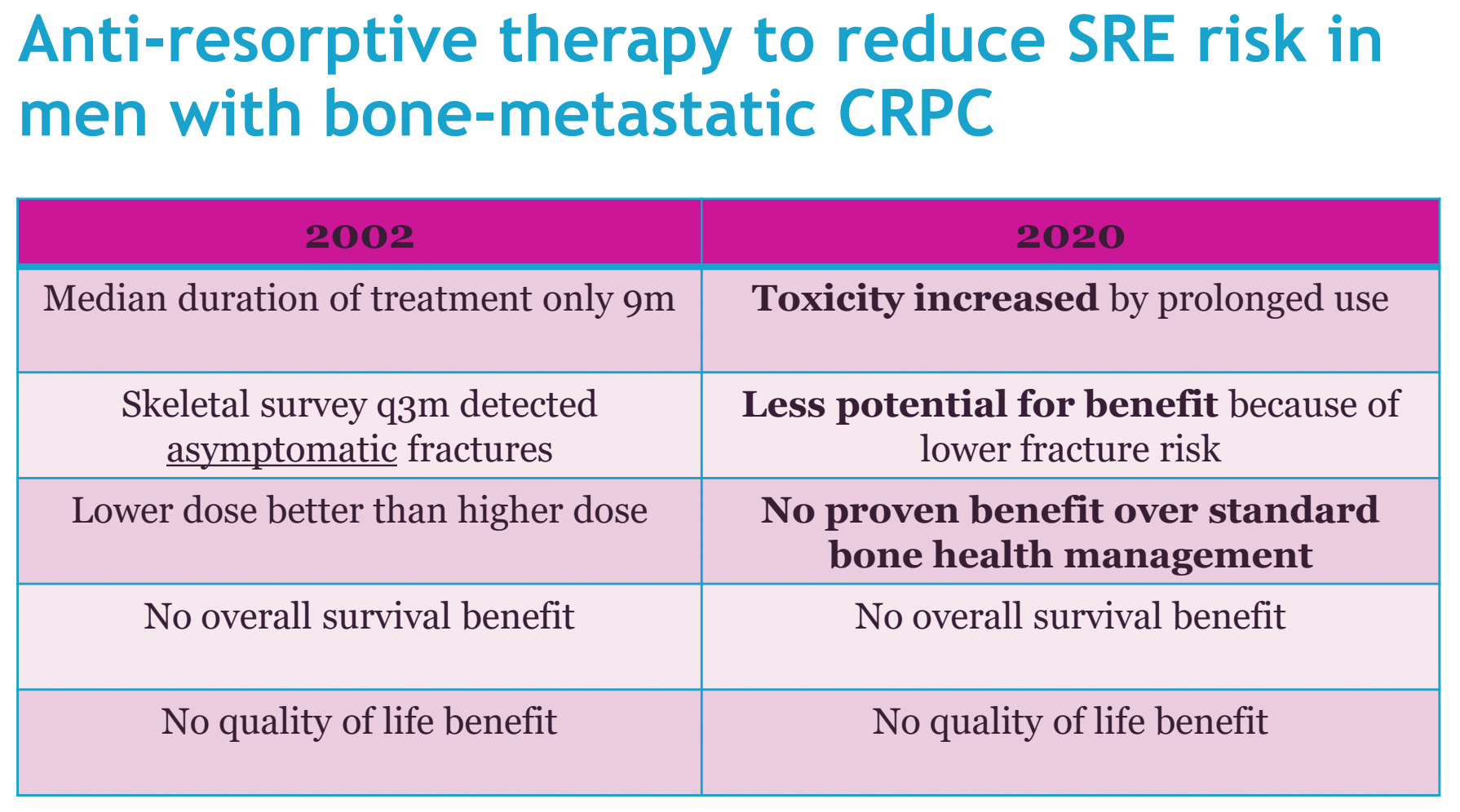
Dr. Parker summarized the evidence base in 2002 after the publication of zoledronic acid 039: (i) the median duration of treatment was only 9 months, (ii) skeletal surveys detected every 3 months detected asymptomatic fractures, (iii) the lower dose was better than the higher dose, (iv) there was no overall survival benefit, (v) there was no quality of life benefit.
Moving forward to 2017, Himelstein and colleagues assessed the effect of longer-interval versus standard dosing of zoledronic acid on skeletal-related events among patients with breast, prostate, multiple myeloma bone metastasis2. For this trial, patients were randomized to receive zoledronic acid administered intravenously every 4 weeks (n = 911) versus every 12 weeks (n = 911) for 2 years. Among these 1,822 patients 795 completed the study at 2 years. A total of 260 patients (29.5%) in the zoledronic acid every 4-week dosing group and 253 patients (28.6%) in the every 12-week dosing group experienced at least one skeletal-related event within 2 years of randomization (risk difference of -0.3%; p < .001 for noninferiority). This trial concluded that the use of zoledronic acid every 12 weeks compared with the standard dosing interval of every 4 weeks did not result in an increased risk of skeletal events over two years.
So, what changed in the last 20 years? According to Dr. Parker, there are several changes, including (i) the mean overall survival of these metastatic patients increasing from 15 months to 32 months, (ii) an increase in the median time to skeletal-related event from 11 months in Dr. Saad’s initial trial in 2002 to 31 months in PREVAIL3, (iii) the type skeletal-related event has changed from a large proportion of fractures in zoledronic acid 039 to primarily radiation therapy to the SRE in PREVAIL, and (iv) an awareness of bone health management.
Dr. Parker notes that a major concern for prolonged treatment for all-comers is osteonecrosis of the jaw for patients on bisphosphonates. As depicted below, the cumulative hazard for this devastating adverse event increases substantially after 3-4 years on the medication:

Even though men are living longer and are on ADT for longer than they ever have before, new drugs for prostate cancer (ie. abiraterone, enzalutamide, etc.) both reduce the risk of skeletal-related events, but with adverse effects on bone health. So, as Dr. Parker notes, bone health is increasingly important, whereas skeletal-related events are less important.
According to the NICE guidelines for bone health in 2017, oral bisphosphonates are recommended as an option for patients if their 10-year probability of osteoporotic fragility fracture risk is at least 1%. The UK Prostate Clinical Excellence Group guidance in 2019 suggested several lifestyle measures for men on ADT, such as weight-bearing exercises, smoking cessation, ≤ 2 units of alcohol per day, and adequate calcium and vitamin D status. They also recommended that men starting ADT for more than 1 year should be considered for oral bisphosphonates.
Dr. Parker concluded with the following summary table depicting differences in bone health management between 2002 and 2020:
Presented by: Chris Parker, MD, MRCP, FRCR, The Royal Marsden Hospital, Surrey, UK
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the 2019 Advanced Prostate Cancer Consensus Conference (APCCC) #APCCC19, Aug 29 - 31, 2019 in Basel, Switzerland
Read the Opposing Debate: Antiresorptive Therapy to Reduce SRE Risk in Men with Bone mCRPC
References:
1. Saad F, Gleason M, Murray, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst 2002 Oct 2;94(19):1458-1468.
2. Himelstein AL, Foster JC, Khatcheressian JL, et al. Effect of longer-interval vs standard dosing of zoledronic acid on skeletal events in patients with bone metastases: A randomized clinical trial. JAMA 2017 Jan 3;317(1):48-58.
3. Loriot Y, Miller K, Sternberg CN, et al. Effect of enzalutamide on health-related quality of life, pain, and skeletal-related events in asymptomatic and minimally symptomatic, chemotherapy-naïve patients with metastatic castration-resistant prostate cancer (PREVAIL): Results from a randomised, phase 3 trial. Lancet Oncol 2015;16(5):509-521.
Further Related Content: Anti-resorptive Therapy to Reduce Skeletal-Related Events Risk in Men with Bone-Metastatic CRPC Presentation
1. Saad F, Gleason M, Murray, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst 2002 Oct 2;94(19):1458-1468.
2. Himelstein AL, Foster JC, Khatcheressian JL, et al. Effect of longer-interval vs standard dosing of zoledronic acid on skeletal events in patients with bone metastases: A randomized clinical trial. JAMA 2017 Jan 3;317(1):48-58.
3. Loriot Y, Miller K, Sternberg CN, et al. Effect of enzalutamide on health-related quality of life, pain, and skeletal-related events in asymptomatic and minimally symptomatic, chemotherapy-naïve patients with metastatic castration-resistant prostate cancer (PREVAIL): Results from a randomised, phase 3 trial. Lancet Oncol 2015;16(5):509-521.
Further Related Content: Anti-resorptive Therapy to Reduce Skeletal-Related Events Risk in Men with Bone-Metastatic CRPC Presentation


