
Dr. Efstathiou notes that PET imaging is highly sensitive for nodal and osseous metastases with sensitivity rates of 98.7%-100%. Importantly, these patients will be upstaged who would otherwise be considered localized or locally advanced due to shortcomings of conventional imaging.
There are several important points with regards to PSMA PET/CT in the initial diagnosis and staging of prostate cancer. First, PSMA PET/CT may guide targeted biopsies for patients and help detect clinically significant prostate cancer. Second, the proPSMA randomized trial1 showed that PSMA PET-CT had a 27% (95% CI 23-31) greater accuracy than that of conventional imaging (92% [88-95] vs 65% [60-69]; p<0.0001). Additionally, this trial found a lower sensitivity (38% [24-52] vs 85% [74-96]) and specificity (91% [85-97] vs 98% [95-100]) for conventional imaging compared with PSMA PET-CT. Furthermore, PSMA PET/CT more frequently resulted in major management changes (28% vs 15%). Third, PSMA PET/CT is a suitable replacement for conventional imaging in men with biopsy-proven, treatment naïve prostate cancer and is FDA approved.
PSMA PET/CT also changes upfront management. First, PSMA PET/CT helps assess lymph node involvement to inform surgical planning, with the caveat that while PSMA PET/CT outperforms conventional imaging in detecting nodal metastases, recent studies report high specificity but moderate sensitivity. Thus, a negative PSMA PET/CT cannot rule out the presence of nodal metastatic disease. Second, definitive radiotherapy is dependent on accurate localization of disease and an adequate dose delivery to appropriate targets, subsequently changing radiation therapy planning. An alteration in definitive radiotherapy fields is estimated to change in 37%-53% of patients after PSMA PET/CT imaging.
For biochemical recurrence, Dr. Efstathiou notes that accurate localization of recurrence is necessary to inform salvage therapies. In this situation, PSMA PET/CT is highly accurate, with a PPV of 0.84 by histopathologic validation, localizing recurrent prostate cancer in 75% of patients. Furthermore, 19%-30% of patients will have at least one PSMA-positive lesion not covered by consensus salvage radiotherapy fields. Prospective studies have shown that clinicians change intended management in 64-68% of patients 2-3.
Dr. Efstathiou notes that the LOCATE trial4 assessed the impact of PET/CT with F-fluciclovine on treatment plans in patients with biochemical recurrence of prostate cancer after primary therapy with curative intent. Among 213 patients in this trial, F-fluciclovine avid lesions were detected in 122 patients (57%). Overall, 126 patients (59%) had a change in management after the scan, which was major in 98 of 126 (78%) and in 88 (70%) were informed by PET/CT findings. The most frequent major changes were from salvage or noncurative systemic therapy to watchful waiting (32 of 126 cases or 25%), from noncurative systemic therapy to salvage therapy (30 of 126 or 24%) and from salvage therapy to noncurative systemic therapy (11 of 126 or 9%). The EMPIRE-1 trial5 published in The Lancet in 2021 evaluated the role of 18F-fluciclovine-PET/CT in improving cancer control compared with conventional imaging (bone scan and either CT or MRI) alone for salvage post-prostatectomy radiotherapy. There were 165 patients randomly assigned in a 1:1 ratio to radiotherapy directed by conventional imaging alone or to conventional imaging plus 18F-fluciclovine-PET/CT. Importantly, 3-year event-free survival was 63.0% (95% CI 49.2-74.0) in the conventional imaging group versus 75.5% (95% CI 62.5-84.6) for 18F-fluciclovine-PET/CT (difference 12.5; 95% CI 4.3-20.8; p=0.0028):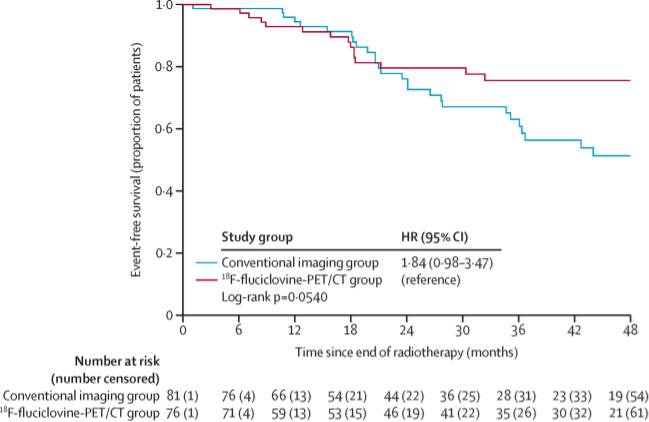
Dr. Efstathiou notes that there are several ongoing studies in this disease space:
- EMPIRE-2: Similar to EMPIRE-1, but patients are randomized to fluciclovine F18 or 68Ga-PSMA PET/CT, allowing dose escalation to regions of PET uptake (n = 140)
- PATRON: An RCT of PSMA PET/CT guided intensification of therapy in patients at risk of prostate cancer (n = 776)
- INDICATE: A phase 3 study of local or systemic therapy intensification directed by PET in prostate cancer patients with post-prostatectomy biochemical recurrence: ECOG-ACRIN EA8191 (n = 894)
- PSMA SRT: A multicenter randomized trial of 68Ga-PSMA-11 PET/CT based SRT after radical prostatectomy (n = 193)
Dr. Efstathiou then moved on to discuss the utilization of PET in identifying oligometastatic disease at initial diagnosis. In the UCSF experience of using PSMA PET/CT prior to definitive radiotherapy, 56% of patients had PET-detected N1 or M1 disease. Thus, we are seeing an expansion of the high-risk/metastatic disease spectrum based on PSMA PET/CT imaging. In the STOMP trial6, Ost and colleagues randomly assigned 62 patients to either surveillance or metastasis-directed therapy of all detected lesions (surgery or stereotactic body radiotherapy), with a primary endpoint of ADT-free survival. At a median follow-up time of 3 years (IQR 2.3-3.75 years), the median ADT-free survival was 13 months (80% CI 12 to 17 months) for the surveillance group and 21 months (80% CI 14 to 29 months) for the metastasis-directed therapy group (HR 0.60, 80% CI 0.40 to 0.90; log-rank p = 0.11). Importantly, metastasis-directed therapy of choline PET CT detected lesions extended time to initiation of ADT compared to observation. The ORIOLE trial7 randomized 54 men in a 2:1 ratio to receive stereotactic body radiotherapy or observation. The primary endpoint for this trial was progression at 6 months, defined as a PSA increase, radiographic or symptomatic progression, ADT initiation, or death. Progression at 6 months occurred in 7 of 36 patients (19%) receiving stereotactic body radiotherapy and 11 of 18 patients (61%) undergoing observation (p = 0.005). Furthermore, treatment with stereotactic body radiotherapy improved median progression-free survival (not reached vs 5.8 months; HR 0.30, 95% CI 0.11-0.81; p = 0.002):
For those patients in the stereotactic body radiotherapy arm that had a PSMA PET-CT scan, the proportion of men with disease progression at 6 months was 5% in those who did not have any untreated lesions, compared to 38% in those who did have some untreated PSMA avid lesions (p=0.03):
In this disease space, there are also several ongoing clinical trials:
- RAVENS: A phase 2 randomized trial of Radium-223 and SABR versus SABR for oligometastatic prostate cancers
- TERPS: A phase 2 trial of total eradiation of metastatic lesions following definitive radiation to the prostate in de novo oligometastatic prostate cancer (systemic therapy + RT +/- SABR)
- STORM: A randomized phase 2 trial for the salvage treatment of oligorecurrent nodal prostate cancer metastases (metastasis-directed therapy + ADT +/- whole pelvis radiotherapy)
Dr. Efstathiou concluded his presentation with the following take-home messages:
- There is a clinically unmet need for more sensitive and specific imaging with improved characterization/localization of clinically relevant tumor burden
- Many studies demonstrate superior diagnostic accuracy of PSMA PET/CT compared to conventional imaging as initial staging and for recurrence
- PET imaging changes clinical decision making, leading to management changes and personalization of therapy
- PET integrated into radiotherapy decision-making/planning significantly improves bEFS and consolidation of all oligometastatic PET avid lesions with metastasis-directed therapy improves PFS and MFS
- As such, we are ready to and have already changed management based on PET imaging
- The stage is set for widespread use of PSMA PET/CT and it is becoming the new standard of care for imaging prostate cancer
- However, with that being said we need (i) more prospective trials with integrated imaging correlates and ideally more RCTs to define the long-term clinical outcome benefits (though recruitment may become more challenging), (ii) reconcile PET findings with trial eligibility, study endpoints and systemic therapy indications, including for nmCRPC, (iii) interpret the results of previous therapeutic trials in a post-PSMA PET setting, (iv) explore PET use as a biomarker to predict response and enable better patient selection, (v) reconcile PET findings with other biomarkers (ie. genomic classifiers), (vi) explore AI and radiomics-based machine learning analysis of PET metrics to further predict disease risk, and (vii) promote standardized imaging interpretation and reporting guidelines
Presented By: Jason Efstathiou, MD, Dana Farber Medical Center, Harvard Medical School, Boston, MA
Written By: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 Advanced Prostate Cancer Consensus Conference (APCCC) Annual Hybrid Meeting, Lugano, Switzerland, Thurs, Apr 28 – Sat, Apr 30, 2022.
References:
- Hofman MS, Lawrentschuk N, Francis, RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomized, multicentre study. Lancet 2020 Apr 11;395(10231):1208-1216.
- Fendler WP, Calais J, Eiber M, et al. Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer: A Prospective Single-Arm Clinical Trial. JAMA Oncol 2019 Jun 1;5(6):856-863.
- Morris MJ, Rowe SP, Gorin MA, et al. Diagnostic Performance of 18F-DCFPyL-PET/CT in Men with Biochemically Recurrent Prostate Cancer: Results from the CONDOR Phase III, Multicenter Study. Clin Cancer Res. 2021 Feb 23 [Epub ahead of print].
- Andriole GL, Kostakoglu L, Chau A, et al. The impact of positron emission tomography with 18F-Fluciclovine on the treatment of biochemical recurrence of prostate cancer: Results from the LOCATE Trial. J Urol 2019 Feb;201(2):322-331.
- Jani AB, Schreibmann E, Goyal S, et al. 18F-fluciclovine-PET/CT imaging versus conventional imaging alone to guide postprostatectomy salvage radiotherapy for prostate cancer (EMPIRE-1): A single centre, open-label, phase 2/3 randomized controlled trial. 2021 May 22;397(10288):1895-1904.
- Ost P, Reynders D, Decaestecker K, et al. Surveillance of Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J Clin Oncol 2018 Feb 10;36(5):446-453.
- Phillips R, Shi WY, Deek M, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol 2020 Mar 26;6(5):650-659.
Related Content: APCCC 2022: Are We Ready to Change Management Based on Next-Generation Imaging? NO


