(UroToday.com) The 2022 Advanced Prostate Cancer Consensus Conference (APCCC) Hybrid Meeting included a session on high-risk and locally advanced prostate cancer and a presentation by Dr. Declan Murphy discussing the impact of histologic variants on treatment recommendations, particularly as it pertains to ductal, intraductal and cribriform histology. Dr. Murphy notes that pathology used to be quite simple, for example 10 years ago histology was scored as Gleason 6-10 and the number of cores were listed. However, today there has been several updates of the ISUP system (2014 and 2019), a grade group system, the percentage of pattern 4 present, the maximum core length, and utilization of genomic classifiers.
The definition of ‘cribriform’ is “cohesive tumor cells with the presence of circular spaces, creating a sieve-like or Swiss cheese appearance.” Furthermore, cribriform glands should be assigned a Gleason pattern 4, regardless of morphology:

Previous studies have shown that cribriform growth is highly predictive for postoperative metastasis and disease-specific death in Gleason 7 prostate cancer, and thus the presence of this growth pattern is important when counseling men about the management of Gleason Group 2 or 3 prostate cancer. Dr. Murphy notes that some favorable intermediate risk cancers are candidates for active surveillance, which in his opinion are men with ISUP 2 (Gleason 3+4 = 7) without cribriform pattern, whereas those with ISUP 2 (Gleason 3+4 = 7) + cribriform pattern are not candidates for active surveillance. Ultimately, it is not well understood why cribriform drives worse outcomes, but it is essential for pathologists to report the presence of cribriform pattern and for clinicians to appreciate the significance of this growth pattern.
With regards to intraductal carcinoma of the prostate, this pattern is an expansive proliferation of malignant cells within pre-existing prostatic ducts and acini with a full or partially conserved basal cell layer with morphological growth similar to the cribriform pattern:1

Work from Dr. Murphy’s group has shown that patient-derived xenografts demonstrate that intraductal carcinoma of the prostate is a prominent pathology in BRCA2 mutation carriers with prostate cancer, which correlates with a poor prognosis.2 This work suggested that intraductal carcinoma of the prostate was present in 42% of BRCA2 patients and only 9% of sporadic patients. As follows is the Kaplan-Meier curve for survival stratified by presence of intraductal carcinoma of the prostate:

A 2017 systematic review from Porter et al.3 assessed intraductal carcinomas of the prostate prevalence with prostate cancer risk. This review, which included radical prostatectomy and prostate biopsy specimens from >7000 patients, showed that the intraductal carcinoma of the prostate prevalence increased from 2.1% in low-risk patient cohorts to 23.1%, 36.7%, and 56.0% in moderate-risk, high-risk, and metastatic or recurrent disease risk categories, respectively (p < 0.0001):

Intraductal carcinoma of the prostate was also highly prevalent in tumours following androgen deprivation therapy or chemotherapy (60%).
Dr. Murphy then posed the question “Should we look for DNA repair defects in the presence of intraductal carcinoma of the prostate”? Velho and colleagues4 assessed pathological and clinical characteristics associated with germline DNA-repair gene mutations, to facilitate selection of patients for germline testing. Among 150 consecutive patients underwent germline testing, pathogenic mutations were identified in 21 men (14%). Among those with germline mutations, 9 (43%) were in BRCA2, 3 (14%) were in ATM, 3 (14%) were in CHEK2, and 2 (9%) were in BRCA1. Men with germline mutations were more likely to harbor intraductal/ductal histology (48% vs 12%, p < 0.01) and lymphovascular invasion (52% vs 14%, p < 0.01). Finally, 44% of patients with a positive germline test would not have been offered genetic screening according to current National Comprehensive Cancer Network (NCCN) guidelines. However, since this study was published, there has been conflicting evidence regarding the role of intraductal carcinoma of the prostate and BRCA mutated prostate cancer. To summarize intraductal carcinoma of the prostate, Dr. Murphy notes that (i) this entity is associated with higher risk prostate cancer, (ii) it may (or may not) be associated with BRCA2 prostate cancer, (iii) this entity is likely still under-reported, and (iv) Dr. Murphy considers this to be like cribriform growth in localized prostate cancer. Ultimately, we should pay attention to the presence of intraductal carcinoma of the prostate, as it is considered to be a marker of aggressiveness.
Ductal adenocarcinoma is defined by its morphology, which includes tall columnar epithelium with stratified nuclei, a papillary, cribriform, or solid architecture, and an absence or patchy basal cell layers:
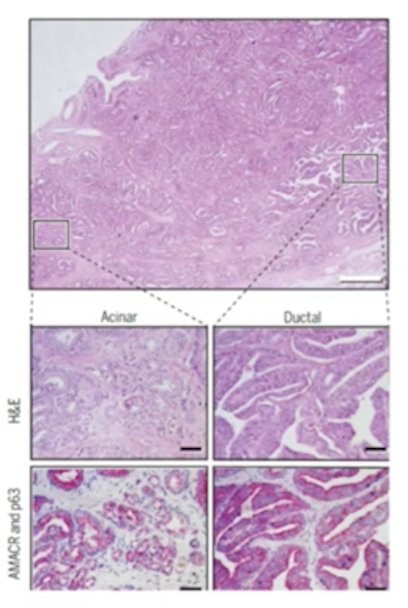
Men with ductal adenocarcinoma often present with lower urinary tract symptoms, and are more likely to present with T3/T4 disease. Additionally, PSA may not be too elevated, there are unusual sites of metastasis, and this histologic variant is associated with a poor prognosis. In a recent paper by Ranasinghe et al.5 at MD Anderson Cancer Center, they showed that ductal prostate cancers demonstrate poor outcomes with conventional therapies. Among 228 men with ductal adenocarcinoma, 63 underwent radical prostatectomy, 34 underwent radiotherapy, and 31 had neoadjuvant therapy prior to radical prostatectomy. Ductal adenocarcinoma patients undergoing radical prostatectomy or radiotherapy had worse 5-year MFS (75% vs 95% and 62% vs 93%, respectively, p < 0.001) and 5-year OS (88% vs 97% and 82% vs 100%, respectively, p < 0.05) compared with prostate adenocarcinoma patients:

In the 76 men who received adjuvant/salvage ADT after radical prostatectomy, ductal adenocarcinoma also had worse MFS and OS than prostate adenocarcinoma (p < 0.01). Additionally, upregulation of several intrinsic resistance pathways among patients with ductal adenocarcinoma renders ADT less effective. To summarize ductal adenocarcinoma, Dr. Murphy notes that it is (i) rare and underreported, (ii) associated with worse stage at presentation (except PSA) (iii) associated with a poor prognosis with local treatment alone, and (iv) associated with a poor response to ADT.
Dr. Murphy concluded his presentation by assessing histological variants in prostate cancer with the following take home messages:
- We need to recognize that pathological variants are important
- Standardized reporting is essential
- We need to work closely with our pathology colleagues
- We need to address pathology questions in clinical trials
Presented By: Declan G. Murphy, FRACS, FRCS, Peter MacCallum Cancer Center, Melbourne, Australia
Written By: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 Advanced Prostate Cancer Consensus Conference (APCCC) Annual Hybrid Meeting, Lugano, Switzerland, Thurs, Apr 28 – Sat, Apr 30, 2022.
References:
- Lawrence MG, Porter LH, Clouston D, et al. Knowing what’s growing: Why ductal and intraductal prostate cancer matter. Sci Transl Med. 2020 Mar 4;12(533):eaaz0152.
- Risbridger GP, Taylor RA, Clouston D, et al. Patient-derived xenografts reveal that intraductal carcinoma of the prostate is a prominent pathology in BRCA2 mutation carriers with prostate cancer and correlates with poor prognosis. Eur Urol. 2015 Mar;67(3):496-503.
- Porter LH, Lawrence MG, Ilic D, et al. Systematic review links the prevalence of intraductal carcinoma of the prostate to prostate cancer risk categories. Eur Urol. 2017 Oct;72(4):492-495.
- Velho PI, Silberstein JL, Markowski MC, et al. Intraductal/ductal histology and lymphovascular invasion are associated with germline DNA-repair gene mutations in prostate cancer. Prostate. 2018 Apr;78(5):401-407.
- Ranasinghe W, Shapiro DD, Hwang H, et al. Ductal prostate cancers demonstrate poor outcomes with conventional therapies. Eur Urol. 2021 Feb;79(2):298-306.


