(UroToday.com) The 2022 Advanced Prostate Cancer Consensus Conference (APCCC) Hybrid Meeting included a session on the management of metastatic hormone sensitive prostate cancer (mHSPC) and a presentation by Dr. Alicia Morgans discussing the treatment of vulnerable/frail patients with mHSPC. Dr. Morgans notes that according to the NCCN guidelines we currently have a plethora of options for the treatment of mHSPC in 2022. However, there is still debate regarding the optimal treatment of newly diagnosed metastatic prostate cancer. Dr. Morgans and Dr. Beltran recently wrote an editorial piece in JCO1 assessing factors that contribute to treatment decisions that should be considered when using shared decision making for patients with mHSPC. This includes cancer-related factors, patient-related factors, clinician-related factors, and treatment-related factors as highlighted in the following figure:

To answer the question “Isn’t androgen deprivation enough?” Dr. Morgans suggests that for the majority of patients the answer is no.
When discussing the vulnerable and frail patient, it is important to define this specific phenotype. Indeed, we live in an aging world, with a predicted 21 to >28% of the population (specifically in Europe and North America) expected to be older than 65 years of age in 2050. Dr. Morgans emphasized that prostate cancer disproportionately affects elderly men, with a median age at diagnosis of 66 years of age and the most frequent age range for diagnosis being 65-74 years of age. However, chronologic and biologic age are not the same.
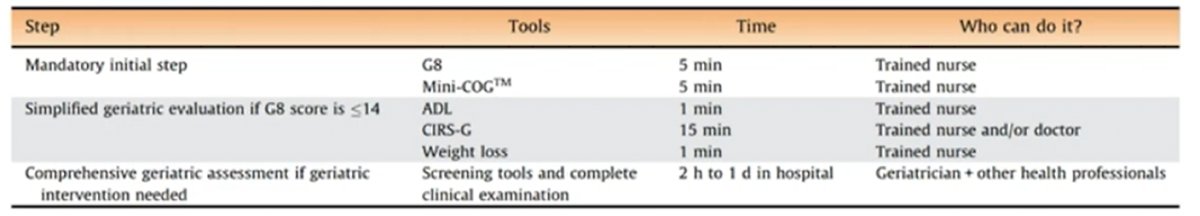
The International Society of Geriatric Oncology has published guidelines for the management of prostate cancer in elderly patients,2 providing key guidance. It is important for clinicians to understand the different steps in health status evaluation and the estimated time required to complete these evaluations. Importantly, a mandatory initial step is to perform a G8 and a Mini-COG assessment:
The G8 screening tool highlights 8 specific items that clinicians address, including those pertaining to food intake, weight loss in the last 3 months, mobility, neuropsychological problems, body mass index, number of daily medications, personal consideration of health status, and age:

The decision tree to determine a patient’s health status is provided by a combination of the G8 and Mini-COG stratifying patients into groups that are fit, frail, or disabled with severe comorbidities. The decision tree for this assessment is as follows:

When assessing disease control outcomes, Dr. Morgans notes that the survival benefit occurs early in follow-up. For example, when looking at abiraterone acetate in the LATITUDE3 and STAMPEDE4 trials, there is early separation of the curves between 6-12 months. Similarly, in the ENZAMET5 and TITAN6 trial, the survival curves separate (favoring the experimental arm) by 9-15 months of follow-up. Dr. Morgans also emphasize that the subgroup analyses for overall survival show a similar benefit regardless of age in the LATITUDE, TITAN, and CHAARTED7 trials. Additionally, there appears to be a potential survival benefit for radiotherapy in low-volume mHSPC in STAMPEDE with relatively low toxicity.8
In a vulnerable/frail patient population, certainly it is important to discuss quality of life outcomes for these patients. Although not analyzed by age, Dr. Morgans notes that quality of life was maintained over time in LATITUDE
Although in the mCRPC disease space, quality of life was also preserved with enzalutamide in the PREVAIL9 trial when analyzed by age.
When looking at adverse events in CHAARTED stratified by age, docetaxel was associated with a small increase in adverse events among patients that were >70 years of age, specifically with regards to neutropenia (age >70: 17% vs age < 60 years: 9%), febrile neutropenia (age >70: 9% vs age < 60 years: 7%) and fatigue (age >70: 9% vs age < 60 years: 2%).
Dr. Morgans concluded her presentation by discussing the treatment of vulnerable/frail patients with mHSPC with the following summary points:
- Standard of care treatment for ALL patients with mHSPC is intensified therapy
- Chronologic and biologic age are not the same; we can use geriatric assessments to help identify areas of reversible decline and areas of need
- Disease control outcomes and quality of life outcomes suggest similar benefits to older and younger patients, with the curves for benefit separating early in follow-up.
Presented by: Alicia Morgans, MD, MPH, Dana Farber Cancer Institute, Harvard Medical School, Boston, MA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 Advanced Prostate Cancer Consensus Conference (APCCC) Annual Hybrid Meeting, Lugano, Switzerland, Thurs, Apr 28 – Sat, Apr 30, 2022.
References:- Morgans AK, Beltran H. Isn’t Androgen Deprivation Enough? Optimal Treatment for Newly Diagnosed Metastatic Prostate Cancer. J Clin Oncol. 2022 Mar 10;40(8):818-824.
- Droz JP, Albrand G, Gillessen S, et al. Management of prostate cancer in elderly patients: Recommendations of a task force of the International Society of Geriatric Oncology. Eur Urol. 2017 Oct;72(4):521-531.
- Fizazi K, Tran N, Fein L, et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med. 2017;377(4):352-360.
- James ND, de Bono JS, Spears MR, et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Engl J Med. 2017;377(4):338-351.
- Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N Engl J Med 2019 Jul 11;381(2):121-131.
- Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med 2019 Jul 4;381(1):13-24.
- Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med. 2015;373(8):737-746.
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): A randomized controlled phase 3 trial. Lancet 2018 Dec 1;392(10162):2353-2366.
- Loriot Y, Miller K, Sternberg CN, et al. Effect of enzalutamide on health-related quality of life, pain, and skeletal-related events in asymptomatic and minimally symptomatic, chemotherapy-naïve patients with metastatic castration-resistant prostate cancer (PREVAIL): Results from a randomised, phase 3 trial. Lancet Oncol 2015;16(5):509-521.


