(UroToday.com) The 2022 Advanced Prostate Cancer Consensus Conference (APCCC) Hybrid Meeting included the Movember session, with a Movember update from Dr. Jane Fisher, and a presentation by Dr. Dan George discussing updates from the IRONMAN Project. Dr. Fisher started by highlighting that Movember has launched a patient centered global cancer real world evidence network with the goal of tackling the following problems:
- There is significant variation in advanced and localized prostate cancer treatment quality leading to major differences in quality of life and survival
- We don’t know what drugs, and in what combinations, work best, and in many cases, we don’t know how to integrate these new drugs with established treatment options
- The quality of life of many men living with prostate cancer is sub-optimal with many experiencing lifelong physical and mental side effects. This includes unacceptable disparities related to poorer health outcomes for various sub-populations based on race, ethnicity, and access to care
- Clinical trials are vital for the development of new tests and new treatments, but recruitment is typically slow and underrepresents non-Caucasian men
Dr. Fisher notes that within 5 years, Movember through the global cancer real world evidence project seeks to have established the leading global prostate cancer real world evidence network that will (i) empower men with the knowledge and tools to take better control of their treatment and its impact throughout the cancer journey to improve their overall quality of life, (ii) significantly reduce the number of men diagnosed with cancer experiencing side effects after treatment by leveraging patient-reported outcome measures (PROMs) within routine care, and (iii) accelerate men’s access to new tests and treatments through access to clinical trials. The goals of this endeavor are to:
- Implement a unified global clinical quality registry that measures and benchmarks outcomes across all disease stages
- Implement personalized digital self-management and telehealth solutions into usual care
- Fund and partner on global biomed research and clinical trials to accelerate new tests and treatments
- Address disparities in access to treatment and care
As follows is the outline for how this initiative works:
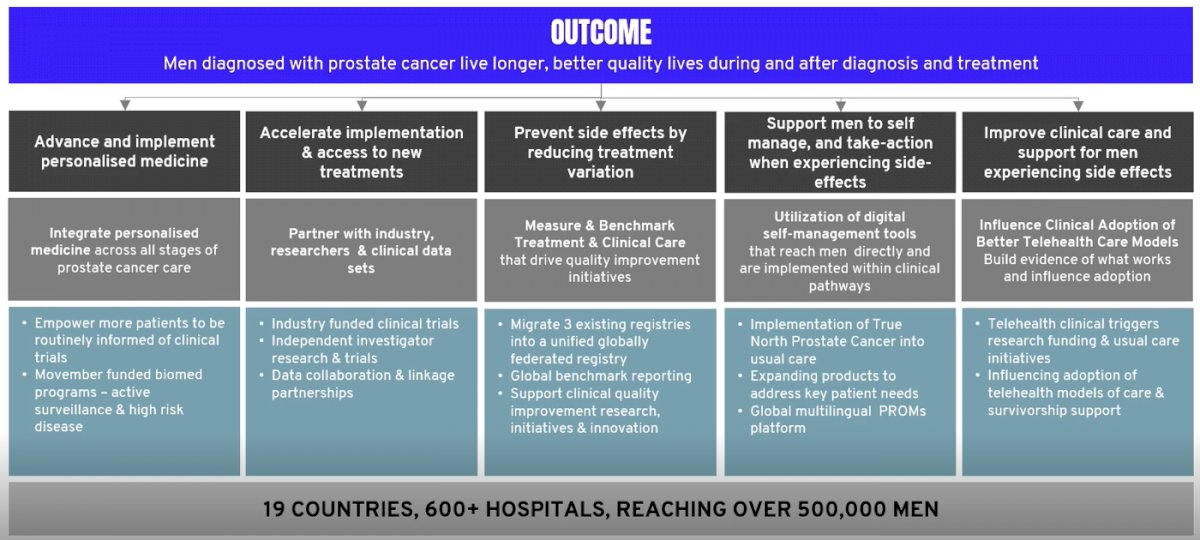
This program will leverage existing data and patient samples to investigate the ways in which existing and/or emerging hormonal or systemic therapies for high-risk, locally advanced prostate cancer or metastatic hormone sensitive prostate cancer can be selected and/or sequenced. The team is a global collaborative team of multidisciplinary investigators to identify the key research question(s) that can be addressed with the program. The expected outcome is a biomarker-informed treatment strategy for men with locally advanced prostate cancer and metastatic hormone sensitive prostate cancer to ensure the right treatment for the right patient at the right time:
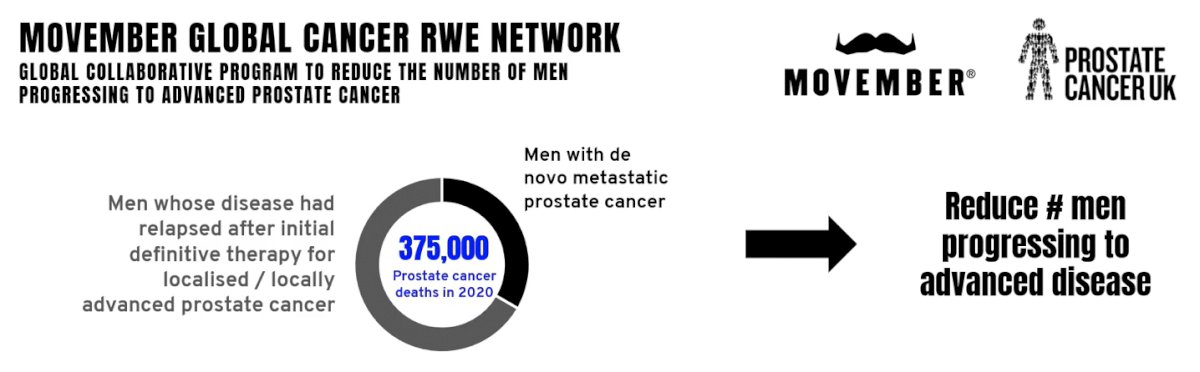
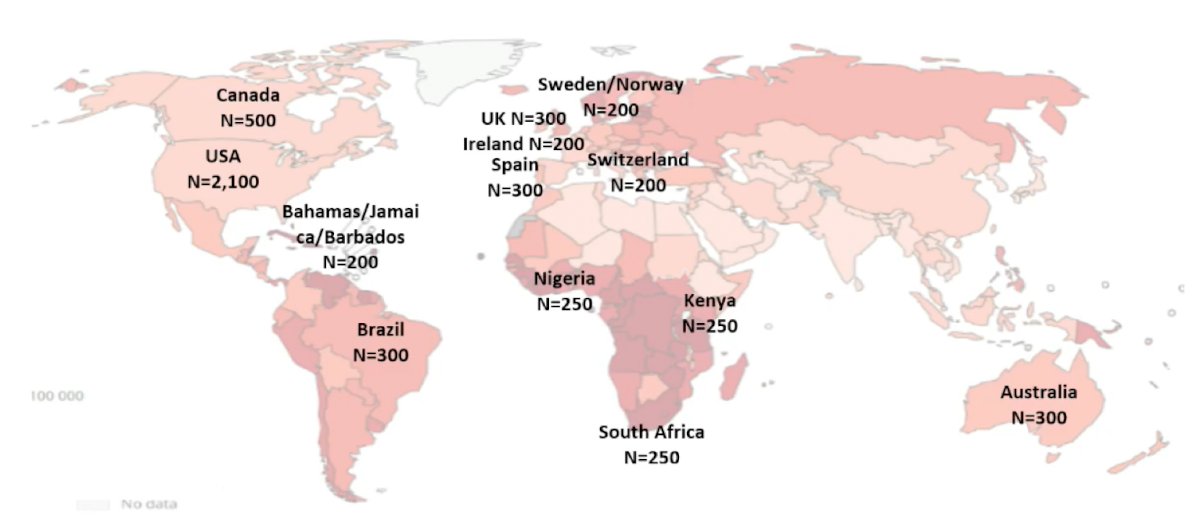
Dr. George then presented an update of IRONMAN, an international registry to improve outcomes in men with advanced prostate cancer. The vision is to create the global IRONMAN registry to (i) understand patient care, experiences, biomarkers, and outcomes among men with advanced prostate cancer in the real-world setting, (ii) identify unmet needs and opportunities for intervention, and (iii) create a resource for the prostate cancer research community. This registry will include several groups of men with prostate cancer, including those with de novo metastatic castration sensitive prostate cancer (mCSPC), men who have progressed to mCSPC after local therapy, and men whose tumors have progressed from mCSPC to castration-resistant prostate cancer. The goal of IRONMAN is to recruit more than 5,000 men with advanced prostate cancer, with the following geographic breakdown to date:
As follows is an overview of the study design, with Dr. George specifically highlighting the physician questionnaires, which will understand reasons for the first change in treatment, subsequent therapy selection, and reasons for treatment discontinuation:
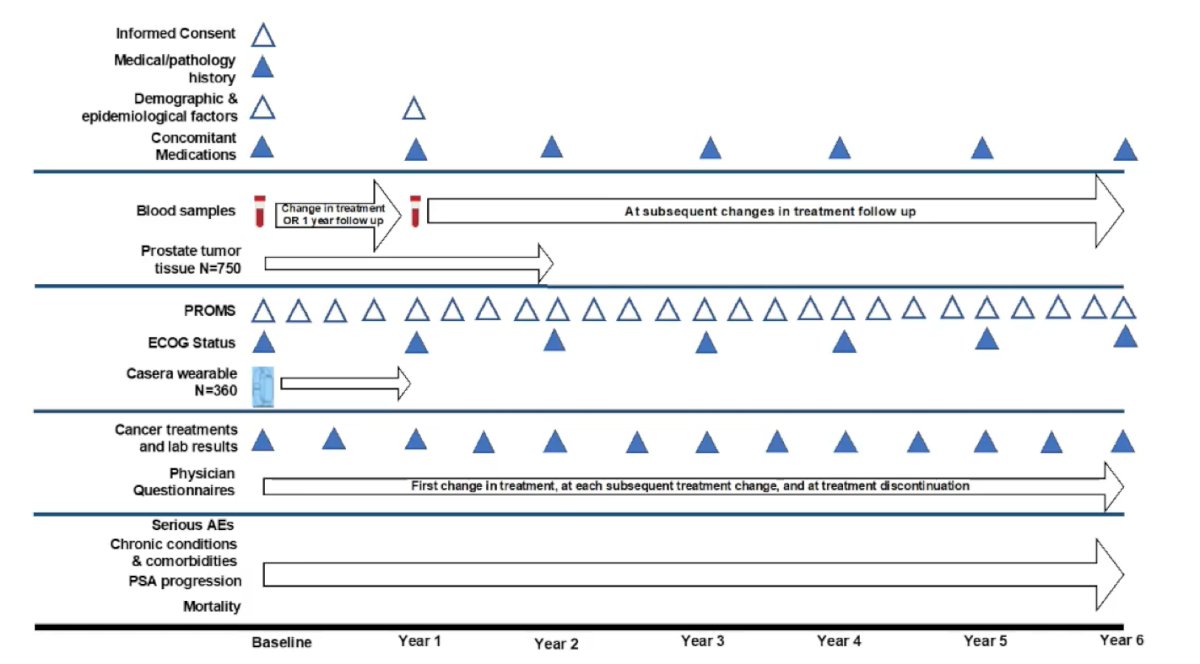
With regards to biospecimens, blood is being collected to perform assays on plasma, whole blood, cfDNA, and RNA. Currently, 37,519 unique specimens have been collected at various time points starting at baseline and all the way through month 24 of treatment. Additionally, tissue is being collected at the time of biopsy and/or at the time of radical prostatectomy. PROMs and patient report experience measures (PREMs) are also being collected with the survey instruments including EORTC QLQ-C30 v3, Brief Pain Inventory (severity, impact, and amount of pain), FACT-FPSI 17, EPIC-26, the Pittsburgh Sleep Quality Index on sleep quality, quantity, disruptions, and medications, Structured Telephone Interview for Dementia Assessment to assess subjective cognitive function, and Cancer Australia’s Core Patient Experience Indicators.
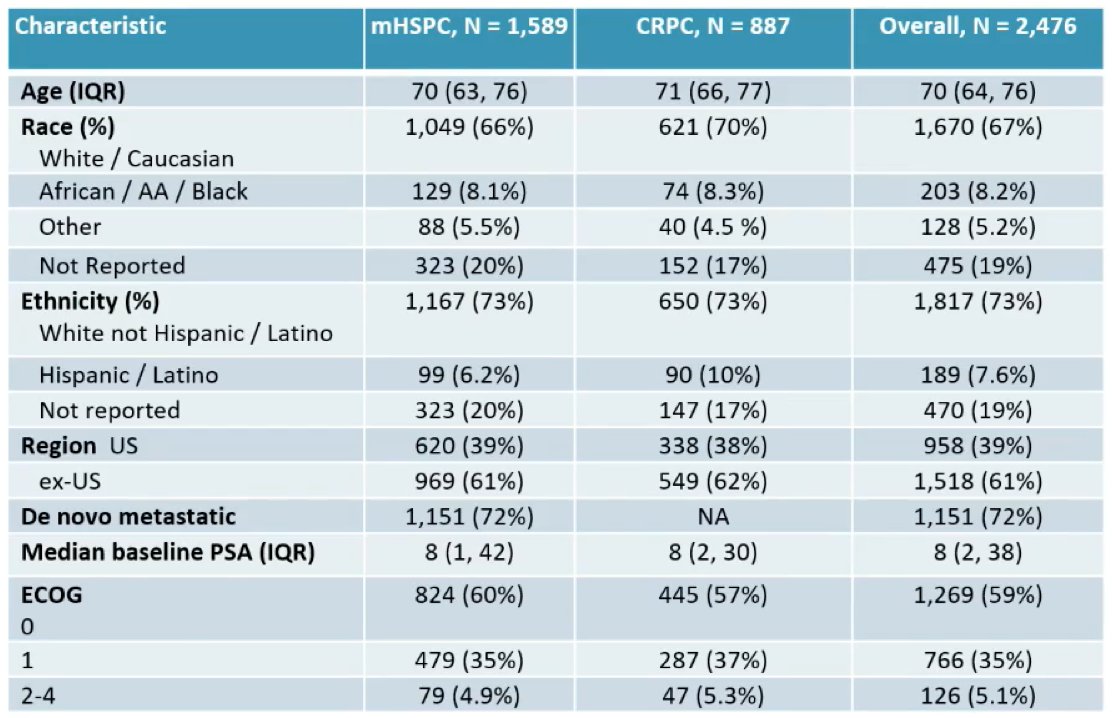
Dr. George notes that based on data presented at ESMO 2021 assessing treatment intensification for mHSPC, nearly 80% of patients in the US Veterans Affairs healthcare system are not receiving treatment intensification with docetaxel or an NHT above and beyond ADT alone. IRONMAN will also be able to look at similar outcomes from a global perspective. The baseline characteristics of the 2,476 men currently in the IRONMAN registry are as follows, highlighting that this registry includes ~8% African American/Black men:
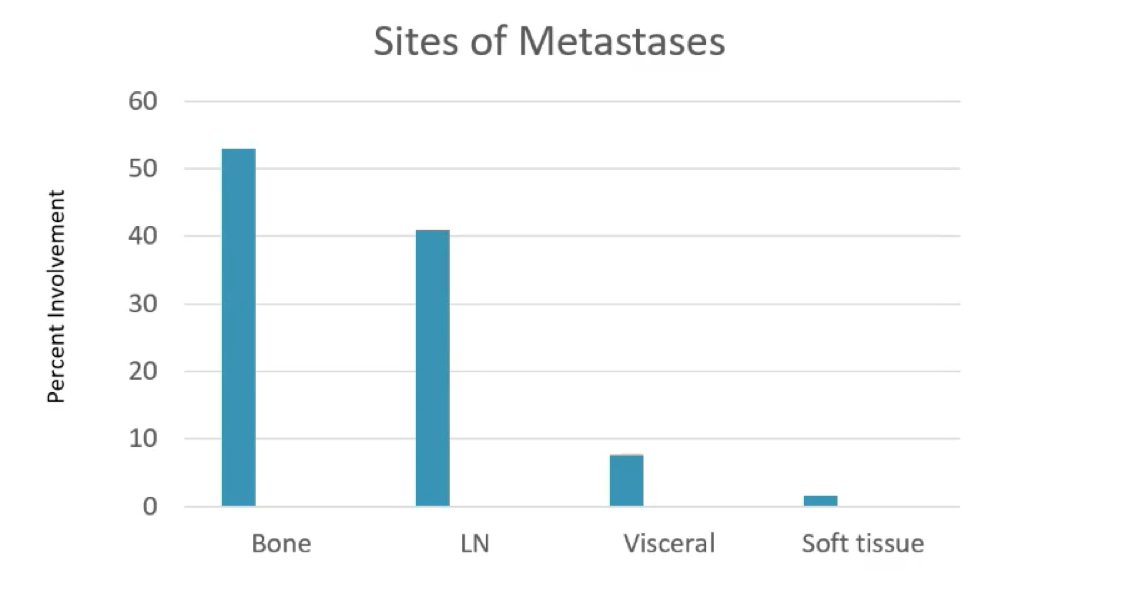
Dr. George then highlighted several interesting preliminary data points, with further assessment and analysis planned for a future congress. For example, more than 50% of men with metastatic disease had bone involvement and over 40% had lymph node involvement:
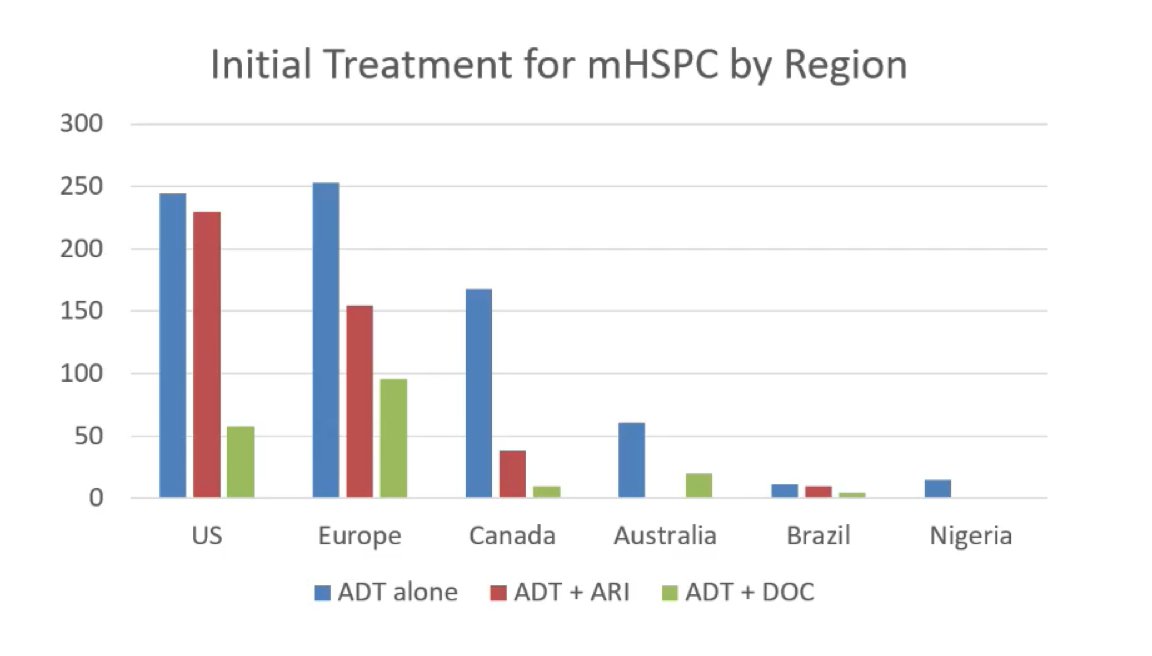
With regards to treatment intensification by geographic region, there were interesting global patterns, particularly with high rates of ADT only utilization in the US, Europe, and Canada:
Several important questions regarding the similarity of IRONMAN participants and their symptom profiles are as follows:
- Does disease status affect quality of life measures?
- Do quality of life measures vary by race/ethnicity?
- Does pain in particular vary by race/ethnicity?
- How are quality of life domains changing over time?
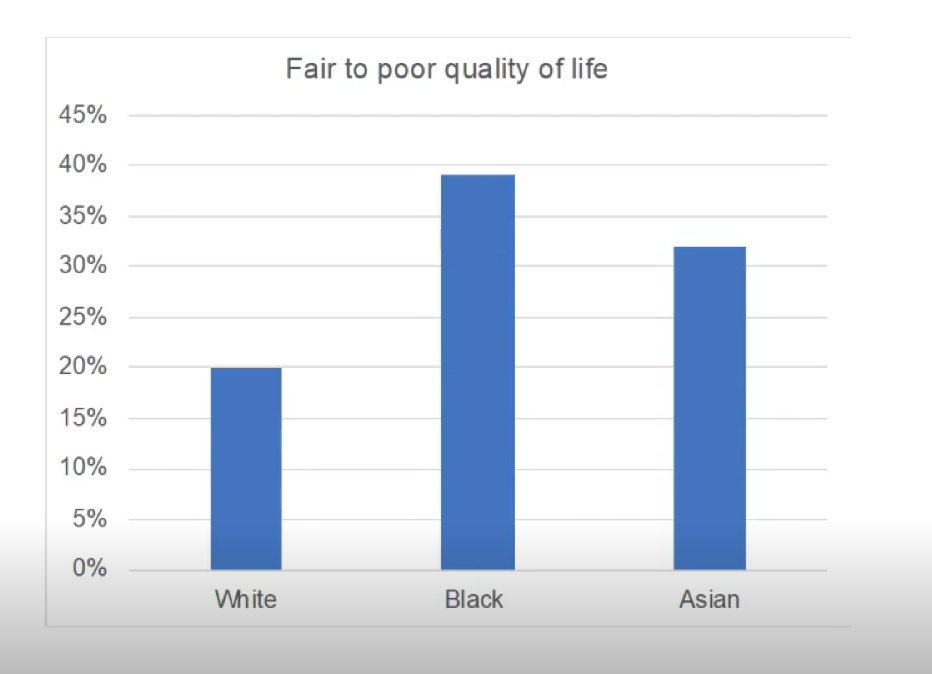
Preliminary data suggest that Black men have a high incidence of fair to poor quality of life in comparison to White and Asian men:
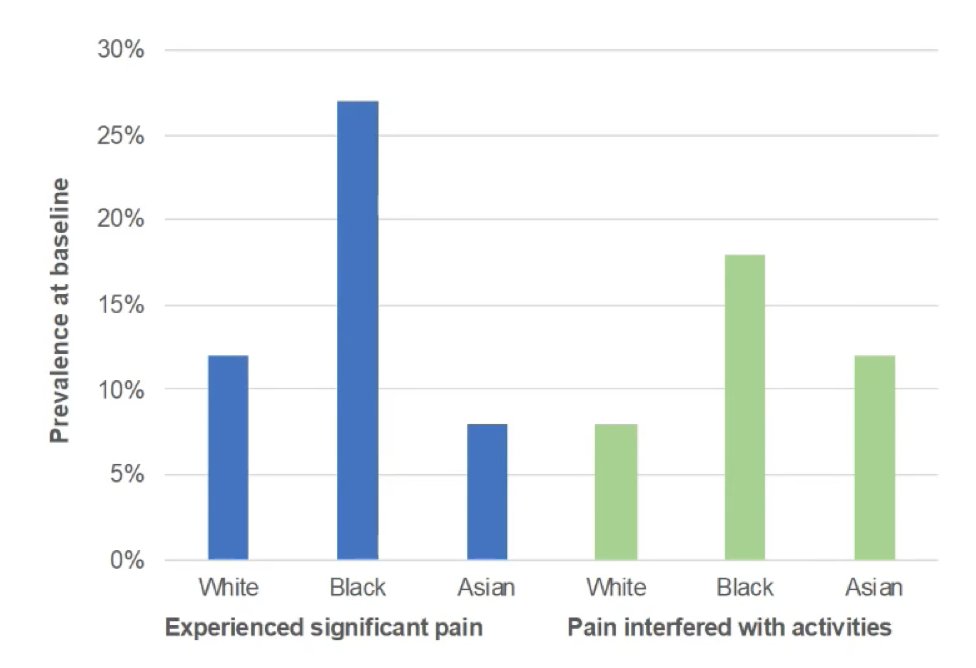
Additionally, black men have higher rates of experiencing significant pain and pain interfering with activities:
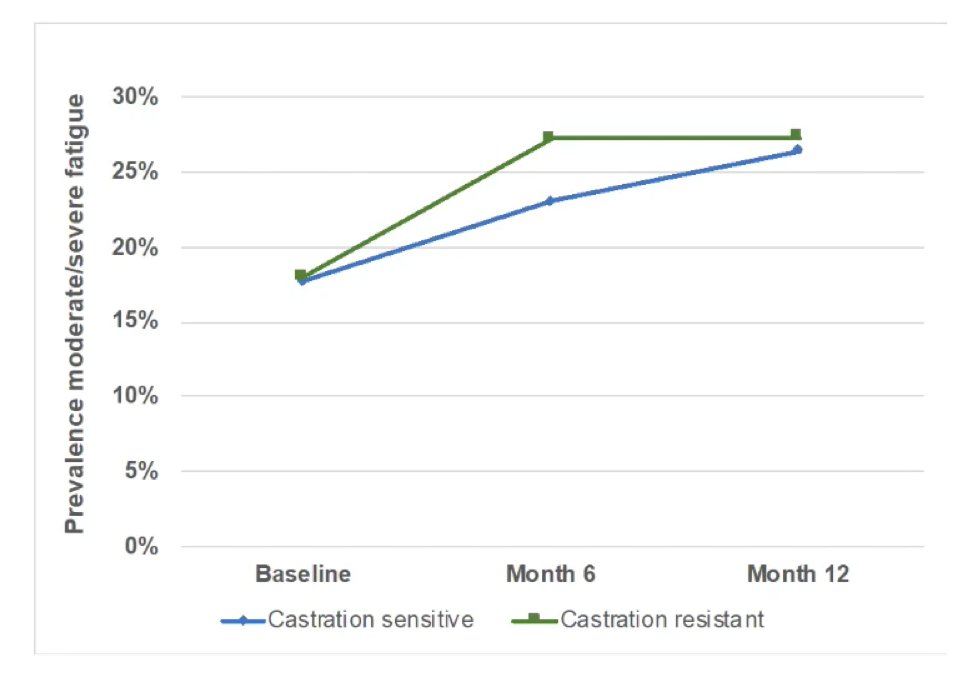
Dr. George also notes that typically we assume that patients have some additional fatigue with treatment, which typically levels off over time, however, preliminary results from IRONMAN suggest that moderate to severe fatigue increases over time:
Dr. George concluded his presentation updating the IRONMAN registry with the following take-home messages:
- IRONMAN is a large, contemporary, international prospective registry in advanced prostate cancer
- It is uniquely designed and supported by investigators, industry, and Advocacy groups
- It will inform practice patterns, gaps in care, and unmet needs
- There is a potential to explore and confirm biomarkers of disease, response, and resistance
- It provides a platform to embed future prospective sub-studies in advanced prostate cancer
Presented by: Jane Fisher, PhD, Global Director Cancer Research and Clinical Trials & Daniel George, MD, Duke Cancer Institute, Durham, NC
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 Advanced Prostate Cancer Consensus Conference (APCCC) Annual Hybrid Meeting, Lugano, Switzerland, Thurs, Apr 28 – Sat, Apr 30, 2022.


