(UroToday.com) The 2022 Advanced Prostate Cancer Consensus Conference (APCCC) Hybrid Meeting included a session on the importance of lifestyle and prevention of complications in advanced prostate cancer and a presentation by Dr. Oliver Sartor discussing how we should take care of our patient’s bones.
Dr. Sartor started by addressing bone issues in the castration sensitive disease setting. First presented at GU ASCO 2022, Dr. Guilhem Roubaud discussed an analysis of bone mineral density in men treated in the PEACE-1 trial. In this trial, dual x-ray absorptiometry (DEXA) was used to assess the bone mineral density (g/cm2) of the lumbar spine, femoral neck, and total hip at baseline, month 6, month 12, and month 24. The authors assessed the mean percentage change in bone mineral density values between baseline and each time point, as well as T-scores. Among the entire PEACE-1 cohort, 210 patients had available bone mineral density data, of whom 182 (87%) had available data at baseline, 109 (52%) had data at month 6, 94 (45%) had data at month 12, and 109 (52%) had data at month 24. Among the 195 patients examined, 97 were treated with abiraterone acetate and prednisone and 98 without.
As shown in the figure below, the mean T score changes in the first 24 months of therapy were essentially superimposable between the two treatment groups.
When examining the change in bone mineral density, there appeared to be a non-significant, early increase in BMD among those patients who received abiraterone. However, by 24 months, the results were nearly interchangeable.
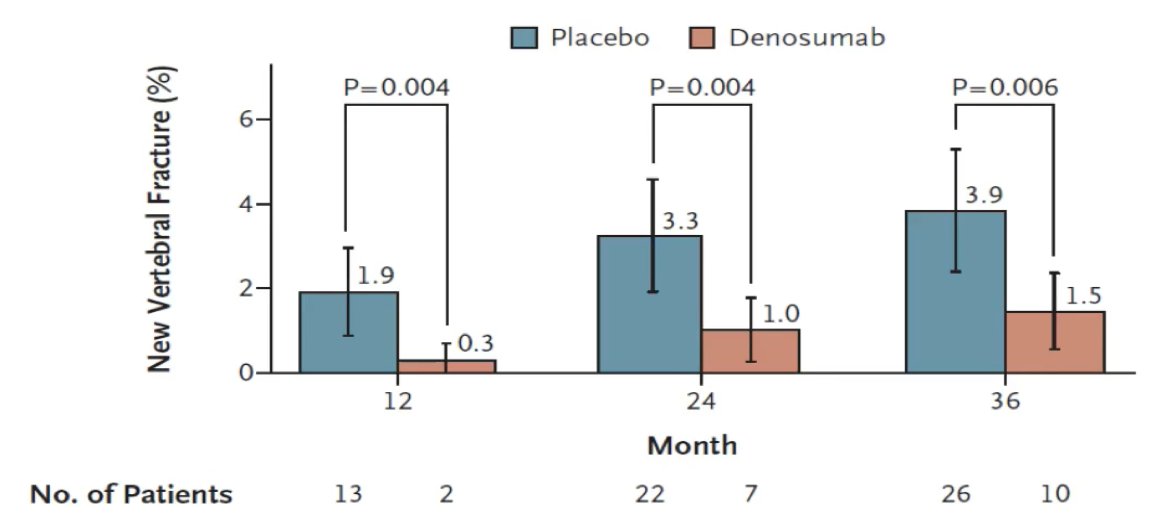
Dr. Sartor then highlighted data from Smith et al.1 looking at the effects of denosumab on bone mineral density and fractures in men receiving ADT for nonmetastatic prostate cancer. In this trial, patients were randomly assigned patients to receive denosumab at a dose of 60 mg subcutaneously every 6 months or placebo (734 patients in each group). At 24 months, bone mineral density of the lumbar spine had increased by 5.6% in the denosumab group as compared with a loss of 1.0% in the placebo group (p < 0.001), and patients who received denosumab had a decreased incidence of new vertebral fractures at 36 months (1.5%, vs. 3.9% with placebo) (relative risk 0.38, 95% CI 0.19 to 0.78; p = 0.006):
Dr. Sartor noted that there are additional interventions that should be discussed with men on ADT, such as supplementation of calcium, vitamin D, exercise, and use of bisphosphonates (for which there are various oral and IV bisphosphonates indicated for osteopenia and osteoporosis). However, Dr. Sartor mentioned that zoledronic acid did not provide a skeletal related event benefit in men with bone metastatic CSPC in the CALGB 90202 trial (zoledronic acid versus placebo p = 0.385).2
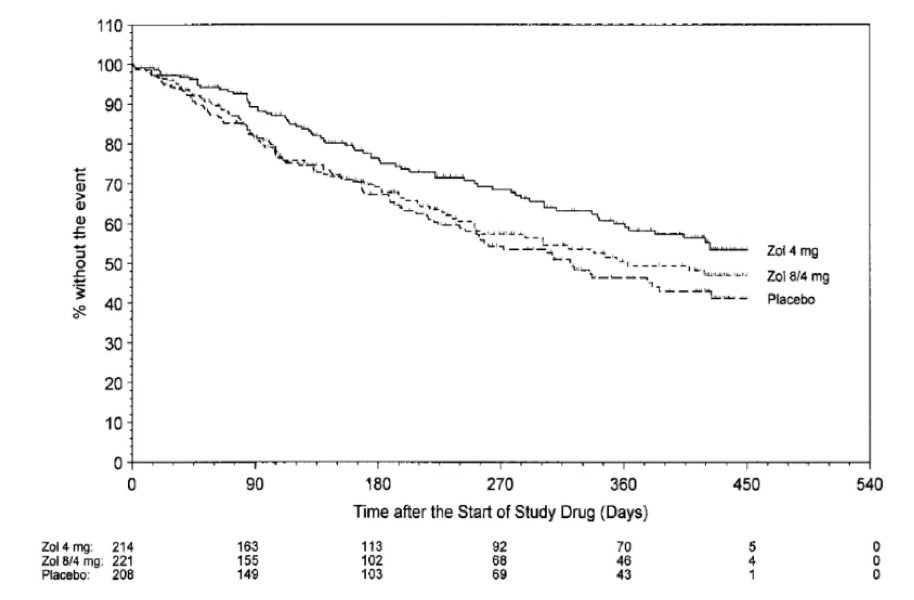
Dr. Sartor then switched gears to discuss bone issues in CRPC, specifically skeletal related events, and symptomatic skeletal events. Data from 20 years ago from Dr. Fred Saad showed that time to skeletal related event was improved with the addition of zoledronic acid among patients with mCRPC, in a dose dependent fashion:3
Additional trials that provided evidence for bone health agents decreasing skeletal related events in patients with mCRPC are as follows:
- Monthly denosumab versus monthly zoledronic acid: HR for skeletal related events 0.82, 95% CI 0.71-0.954
- Skeletal related event rate reduced by effective therapy in mCRPC (abiraterone + prednisone vs placebo + prednisone in the COU-AA-301 trial): HR for skeletal related event 0.615, 95% CI 0.478-0.7915
- Skeletal related event rate reduced by effective therapy in mCRPC (enzalutamide vs placebo in the PREVAIL trial): HR for skeletal related event 0.72, 95% CI 0.61-0.846
- Skeletal related event rate reduced by effective therapy in mCRPC (radium-223 vs placebo in the ALSYMPCA trial): HR for skeletal related event 0.66, 95% CI 0.52-0.837
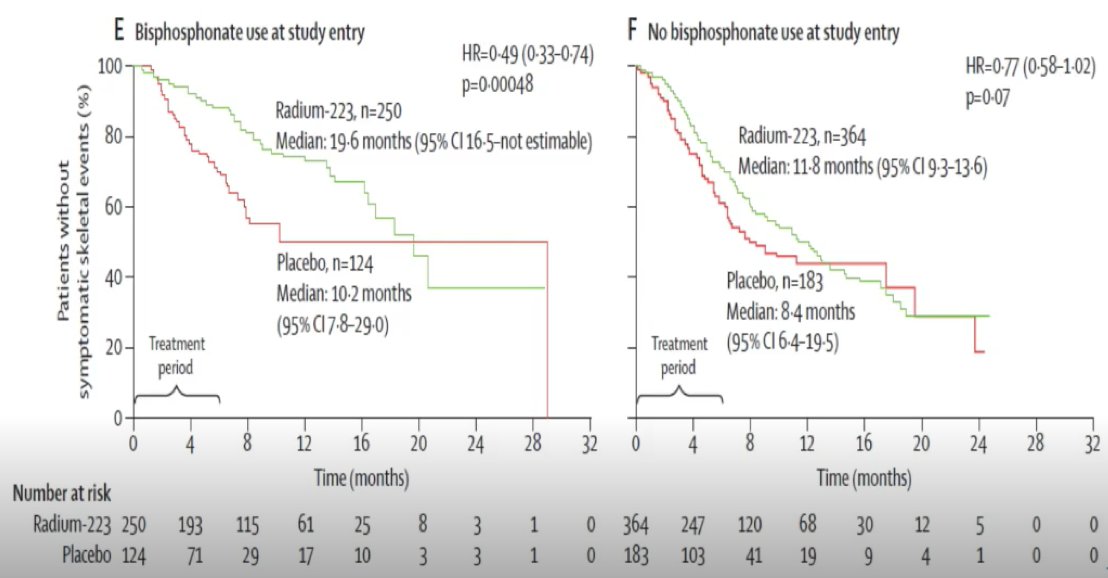
Dr. Sartor also discussed the impact of radium-223 + bisphosphonate use at the time of entry based on additional analyses from the ALSYMPCA dataset,8 noting a substantial improvement in time to skeletal related event with the use of both agents:
ERA 223 was a phase 3 trial that randomized 806 patients with chemotherapy-naïve, mCRPC with bone metastasis to radium-223 or placebo, in addition to abiraterone acetate.9 Symptomatic skeletal event-free survival was the primary outcome. The trial was unblinded prematurely as more fractures and deaths were identified in the radium-223 arm than among patients receiving placebo. Median skeletal event-free survival was 22.3 months (IQR 17.0 to 25.8 months) among patients receiving radium-223 and abiraterone acetate and 26.0 months (IQR 21.8 months to 28.3 months) in patients receiving placebo and abiraterone acetate (HR 1.12, 95% CI 0.92-1.37). Fractures were more common among patients receiving radium-223 and abiraterone acetate (29%) than those receiving placebo and abiraterone acetate (11%), and time to first fracture significantly favored the placebo arm:

In parallel to the ERA-223 trial, the randomized phase III EORTC-1333-GUCG trial compared enzalutamide versus a combination of radium 223 and enzalutamide in men with asymptomatic or mildly symptomatic mCRPC. Based on the early unblinding of ERA-223 due to a significant increase in the rate of fractures in the combination of abiraterone and radium-223, this led to an amendment in the EORTC-1333-GUCG trial to mandate use of bone protecting agents. Updated results of this safety analysis were presented by Dr. Gillessen at ASCO 2021, with a median follow-up of 36.7 months in patients prior to the amendment mandating bone protective agents and 23.1 months among those patients receiving bone protective agents, a total of 39 patients reported a fracture. The vast majority of these events (30 patients: 20 in the enzalutamide + radium-223 and 10 in the enzalutamide only arm) occurring in patients not receiving bone protective agents while 9 events occurred on those receiving bone protective agents (4 of which occurred in patients receiving enzalutamide and radium-223).
Dr. Sartor emphasized that various studies continue to indicate that bone health agents are underutilized in all settings. For example, even with the recent VISION trial publication,10 only 56% of patients received concomitant bone health agents, with various real-world evidence studies from multiple countries showing similar data.
Recently, there has been concern regarding an increased risk of vertebral fracture after stopping denosumab, which may lead to multiple fracture that may occur as soon as 8 months after stopping the medication. Another concern is that patients without risk of osteoporotic fracture are receiving bone-modifying agents. In a recent study by Mitchell et al.,11 it was estimated that among men with mCSPC who had no evidence of high osteoporotic fracture risk, more than one-quarter have received bone-modifying agents in recent years, which may lead to excess costs and toxicity.
Dr. Sartor notes that there is also little data on dosing and schedule for patients receiving zoledronic acid and denosumab. For patients with osteopenia, the standard is zoledronic acid 5 mg/year and denosumab 60 mg every 6 months. For skeletal related event prevention, the standard is zoledronic acid 4 mg every 3-4 weeks and denosumab 120 mg every 4 weeks, but there are no clear dose/schedule comparisons. The CALGB 70604 and OPTIMIZE-2 trials showed that zoledronic acid every 3 months was not inferior to every month dosing, and the REaCT-BTA trial showed similar outcomes with denosumab or bisphosphonates dosed every 1 or 3 months.
Dr. Sartor concluded this portion of his presentation with the following summary points:
- Bone health agents continue to be under utilized
- Denosumab cessation is associated with rebound risk
- Overuse of skeletal related event agents persists in castration sensitive prostate cancer
- Dosing intervals are not fully resolved
Presented by: Oliver Sartor, MD, Tulane University, New Orleans, LA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 Advanced Prostate Cancer Consensus Conference (APCCC) Annual Hybrid Meeting, Lugano, Switzerland, Thurs, Apr 28 – Sat, Apr 30, 2022.
References:- Smith MR, Egerdie B, Toriz NH, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745-755.
- Smith MR, Halabi S, Ryan CJ, et al. Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: Results of CALGB 90202 (Alliance). J Clin Oncol 2014;32(11):1143-1150.
- Saad F, Gleason M, Murray, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst 2002 Oct 2;94(19):1458-1468.
- Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomized, double-blind study. Lancet 2011;377(9768):813-822.
- Logothetis CJ, Basch E, Molina A, et al. Effect of abiraterone acetate and prednisone compared with placebo and prednisone on pain control and skeletal-related events in patients with metastatic castration-resistant prostate cancer: Exploratory analysis of data from the COU-AA-301 randomized trial. Lancet Oncol. 2012 Dec;13(12);1210-1217.
- Loriot Y, Miller K, Sternberg CN, et al. Effect of enzalutamide on health-related quality of life, pain, and skeletal-related events in asymptomatic and minimally symptomatic, chemotherapy-naïve patients with metastatic castration-resistant prostate cancer (PREVAIL): Results from a randomised, phase 3 trial. Lancet Oncol 2015;16(5):509-521.
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369(3):213-223.
- Sartor O, Coleman R, Nilsson S, et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: Results from a phase 3, double-blind, randomized trial. Lancet Oncol. 2014 Jun;15(7):738-746.
- Smith M, Parker C, Saad F, et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(3):408-419.
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021 Sep 16;385(12):1091-1103.
- Mitchell AP, Meza AM, Panageas KS, et al. Real-world use of bone-modifying agents in metastatic castration-sensitive prostate cancer. J Natl Cancer Inst. 2022 Mar 8;114(3):419-426.


