(UroToday.com) In the session of the 2022 Advanced Prostate Cancer Consensus Conference focusing on the treatment of patients with metastatic castration-resistant prostate cancer (mCRPC), Dr. Maha Hussain discussed the optimal treatment sequencing in mCRPC.
She began by highlighting the remarkable changes we have seen in the treatment of mCRPC over the past 20 years or so. While there were no proven life-prolonging agents prior to 2004, we now have a plethora of treatment choices that place us in the enviable situation of having to consider optimal sequencing approaches.

Dr. Hussain emphasized that there are two core principles in systemic therapy in oncology: when modalities are independently effective, combination therapy should be explored, and those active agents should be advanced earlier in the disease trajectory. Today, combination treatment approaches have not been approved for treatment in mCRPC. However, there are multiple circumstances of treatment sequencing, including androgen receptor targeting therapies, cabazitaxel, and olaparib.
She first addressed the question of sequencing androgen receptor targeting therapies, citing randomized phase II data from Drs. Khalaf and Chi. In a randomized fashion, they compared the outcomes of abiraterone acetate followed by enzalutamide or enzalutamide followed by abiraterone. Dr. Hussain emphasized that the sequence of abiraterone followed by enzalutamide had better outcomes, though the overall clinical benefit of the second oral agent was relatively muted. She noted that the underlying biology to explain this observation is not well understood. Further, it is unclear whether prior treatment exposure during the metastatic castration sensitive prostate cancer disease space will affect responses in the mCRPC setting.
She then moved to discuss treatment options in the third-line space, based on data from the CARD trial. This trial enrolled patients with mCRPC who have prior docetaxel and either abiraterone or enzalutamide. They were randomized to receive either a switch in OR agent or cabazitaxel.
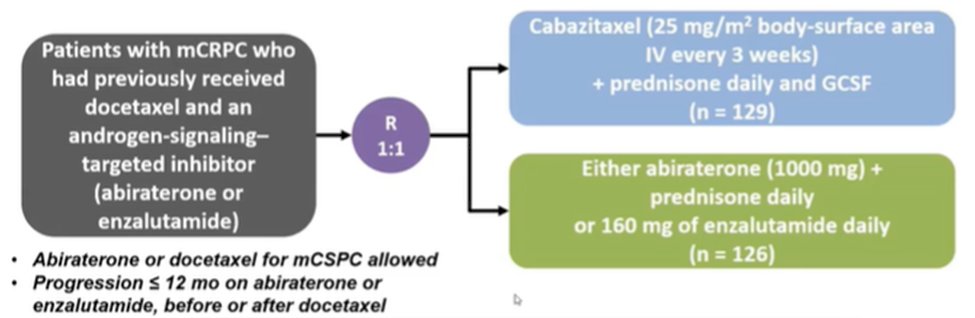
Dr. Hussain noted that patients treated with cabazitaxel had better radiographic progression-free survival, overall survival, and progression-free survival. Thus, she emphasized that it is important to “properly choose the drugs”. However, among asymptomatic patients who are slowly progressing, she suggested that it is “not completely wrong” to consider an AR inhibitor switch.
She further presented data from a post hoc analysis of PROfound. This trial included a mostly heavily pretreated population, though not everyone had seen chemotherapy. In this post hoc analysis, there appeared to be some mutation dependent effect modification by receipt of prior taxane therapy on the efficacy of olaparib. For patients with BRCA1/2 mutations, olaparib response was independent of prior taxane exposure. However, for patients with CDK12 mutations, there were better outcomes from olaparib therapy among those patients without prior taxane exposure while those patients with ATM mutations had better response to olaparib if they had prior taxane treatment.
Moving forward, Dr. Hussain emphasized that there are currently many trials assessing combinatorial treatment approaches. Among these, the phase III PROpel trial assessing the combination of olaparib and abiraterone and the phase III MAGNITUDE trial of niraparib and enzalutamide were recently reported at GU-ASCO 2022.
She first discussed the PROpel which was not biomarker stratified. In spite of this, PROpel was a positive trial for the primary outcome of radiographic progression-free survival. In contrast, MAGNITUDE was a biomarker stratified trial which showed a benefit of the combination approach in the biomarker positive subset, while it was negative in the biomarker negative subset which was closed due to futility. Dr. Hussain then considered how approval of this combination approach will affect downstream options.
She then considered how treatment changes in the mCSPC disease space affect mCRPC treatment options. Since 2015, we have seen evidence of the benefit of docetaxel, abiraterone acetate, apalutamide, and enzalutamide in mCSPC. More recently, PEACE-1 and ARASENS support the use of combination therapy with ADT + docetaxel and either abiraterone or darolutamide. PEACE-1 showed evidence of the benefit of this combination approach, compared to docetaxel + ADT in both the low-volume and high-volume setting, though median overall survival was not reached in the low-volume subset.

Similarly, ARASENS showed improvements in overall survival, as well secondary endpoints such as castration-resistant prostate cancer. The question, therefore, is how to manage treatment progression.
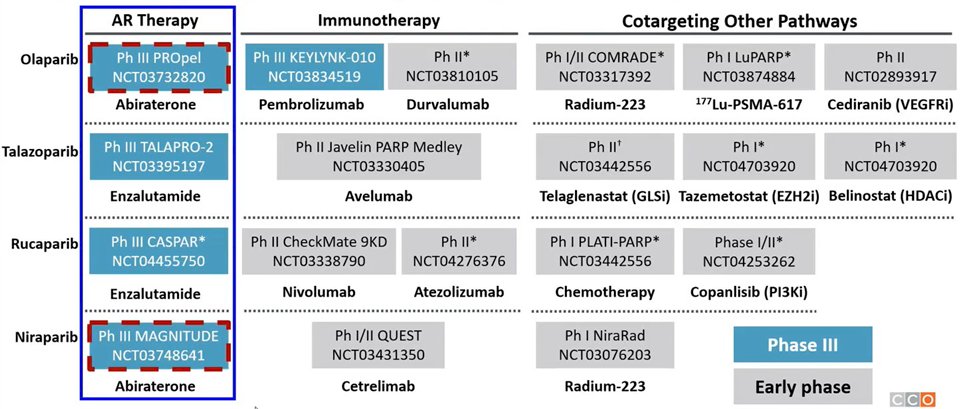
Dr. Hussain then noted that there are many ongoing studies examining the role of PARP inhibitors in earlier disease spaces, as highlighted in the figure below.

Further, using a slide from Dr. Aggarwal, Dr. Hussain emphasized that there has been significant improvement in outcomes for patients over the past decade. She noted that the median overall survival of patients receiving ADT alone in the control arm treated in CHAARTED (44 months) was substantially shorter than that seen in the recent SWOG 1216 disease (70 months). This shows evidence of the effect of downstream treatment options in mCRPC changing outcomes for trials in mCSPC.
When considering options in mCRPC, she considered not just the importance of prior therapy in mCSPC or nmCRPC but also patient characteristics (including performance status, comorbidities, and symptoms), patient preferences, genomic features, and health system factors (such as availability, cost, and logistics).
Concluding, Dr. Hussain emphasized that there has been tremendous progress in the management of patients with nmCRPC, mCSPC, and mCRPC. This has resulted in complex considerations regarding treatment sequencing. While moving effective therapy to earlier disease states has a better “return on investment”, we need to better understand how this affects response to in-class agents and different agents in mCRPC.
Presented by: Maha Hussain, MD, FACP, FASCO, Genevieve Teuton Professor of Medicine in the Division of Hematology-Oncology, Department of Medicine, and the Deputy Director, and leader of the GU Oncology Program at the Robert H. Lurie Comprehensive Cancer Center of the Northwestern University Feinberg School of Medicine

