(UroToday.com) The 2022 Advanced Prostate Cancer Consensus Conference (APCCC) Hybrid Meeting included a session on the management of non-metastatic castrate-resistant prostate cancer (nmCRPC) and a presentation by Dr. Joaquin Mateo discussing genomic and clinical markers in this disease space. Dr. Mateo notes that nmCRPC may or may not be traversed as patients pass through the prostate cancer disease continuum, as highlighted in the following figure:

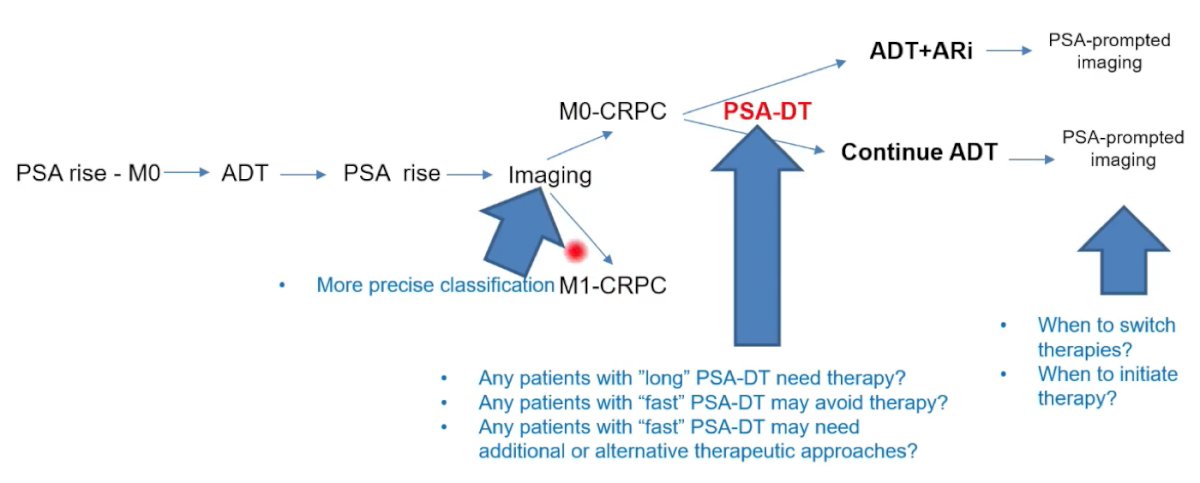
There are several potential areas of improvement where biomarkers may provide help. First, imaging in the setting of PSA rise depicts whether patients are M0 CRPC or M1 CRPC, thus there may be a potential role for biomarkers improving precise classification. Second, the important aspect of PSA doubling time is a potential area where biomarkers may be of assistance. Several important questions with regards to the utility of PSA doubling time are as follows:
- Do patients with “long” PSA doubling times need therapy?
- Are there any patients with “fast” PSA doubling times that may avoid therapy?
- Are there any patients with “fast” PSA doubling times that may need additional or alternative therapeutic approaches?
Third, biomarkers may be able to assist with improving care after treatment when PSA prompts imaging. In this setting, important questions arise with regards to when to switch therapies and/or when to initiate therapy:
Feng et al.1 previously looked at the association of molecular subtypes with differential outcomes to apalutamide treatment in men with nmCRPC. In this study, gene expression data from 233 archived samples from patients enrolled in the SPARTAN trial were generated using a human exon microarray. Patients were subsequently stratified into high-risk and low-risk categories for developing metastases based on genomic classifier (GC) scores for high (GC >0.6) and low to average (GC≤0.6), and into basal and luminal subtypes. Having high GC scores was associated with the greatest improvement in MFS (HR 0.21, 95% CI 0.11-0.40), OS (HR 0.52, 95% CI 0.29-0.94), and PFS2 (HR 0.39, 95% CI 0.23-0.67) vs placebo + ADT. Patients with luminal subtype in the apalutamide + ADT arm had a significantly longer MFS (apalutamide + ADT: HR 0.40, 95% CI 0.18-0.91; placebo + ADT: HR 0.66, 95% CI 0.33-1.31) compared with patients with basal subtype. Additionally, similar trends were observed for OS (apalutamide + ADT: HR 0.50, 95% CI 0.25-0.98; placebo + ADT: HR 0.78, 95% CI 0.38-1.60), and PFS2 (apalutamide + ADT: HR 0.71, 95% CI 0.42-1.22; placebo + ADT: HR 0.72, 95% CI 0.38-1.39). As follows is the Kaplan-Meier curves for the association of Decipher risk scores with MFS:

Dr. Mateo notes that with regards to treatment-induced selective pressure and prostate cancer evolution, perhaps it possible that genomic profiling can better stratify patients with nmCRPC. However, (i) what is the genomic make-up of nmCRPC? and (ii) is the genomics of nmCRPC the same as genomics of mCRPC? Perhaps liquid biopsy could help improve management of nmCRPC patients. In work presented at ASCO 2020, men in the TITAN study that progressed from mHSPC to mCRPC had positive ctDNA at baseline in 23.7% of cases, which increased to 63.6% among those patients that progressed. Men in the SPARTAN study that progressed from nmCRPC to mCRPC had positive ctDNA at baseline in 7.5% of cases, which increased to 27% among those patients that progressed.
Dr. Mateo concluded his presentation by discussing genomic and clinical markers in nmCRPC with the following take-home messages:
- Therapeutic indication for ADT versus ADT + ARI in nmCRPC is based on prognostic (imaging + PSA doubling time) markers; further research could identify others (including clinical variables, PSA kinetics, pathology features) that could help refine stratification
- There is no data on potential predictive biomarkers (+/-) for ARIs in this space, and no studies for drugs with associated predictive biomarkers (alone or in combination)
- Functional imaging is likely to redefine the setting by re-classifying the disease
- Genomic biomarkers have prognostic value in localized prostate cancer and in mHSPC/nmCRPC
- We need to understand biological differences between tumors that evolve towards nmCRPC versus those that evolve towards mCRPC up front
- Transcriptomic profiling seems to have prognostic value in nmCRPC
- Liquid biopsy could provide an additional measure of tumor burden, including more accurate disease evaluation, and compliment imaging for monitoring response and disease progression
Presented by: Joaquin Mateo, MD, Vall d’Hebron Institute of Oncology, Barelona, Spain
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 Advanced Prostate Cancer Consensus Conference (APCCC) Annual Hybrid Meeting, Lugano, Switzerland, Thurs, Apr 28 – Sat, Apr 30, 2022.
References:
- Feng FY, Thomas S, Saad F, et al. Association of molecular subtypes with differential outcome to apalutamide treatment in nonmetastatic castration-resistant prostate cancer. JAMA Oncol. 2021;7(7):1005-1014.


