(UroToday.com) In the session of the 2022 Advanced Prostate Cancer Consensus Conference focusing on the treatment of patients with metastatic castration-resistant prostate cancer (mCRPC), Dr. Small discussed aggressive variant prostate cancer and treatment-associated small cell/neuroendocrine prostate cancer (t-SCNC).
To begin, he noted that primary small cell prostate cancer is very rare, representing only 1% of newly diagnosed prostate cancer. These patients often present with widely disseminated metastatic disease with visceral metastases commonly identified. Classically, these patients are non-PSA producing, with an androgen-receptor null phenotype. As a result, these patients are often not responsive to androgen-receptor targeted therapies and have a poor prognosis. In contrast, to primary small cell prostate cancer, treatment emergent small cell / neuroendocrine prostate cancer (t-SCNC) is an emerging resistance phenotype among patients initially diagnosed with adenocarcinoma.
Aggressive variant prostate cancer is a phenotypic syndrome including a number of poor-risk features in addition to small cell prostate cancer. These include exclusively visceral metastases, radiographically predominantly lytic metastases, bulky nodal or local disease, low PSA secretion, neuroendocrine markers on histology or serum, or a short-interval to CRPC following the initiation of ADT. He noted that, among these, histologic evidence of small cell prostate carcinoma, bulky lymphadenopathy or a bulky high-grade primary tumor, and neuroendocrine markers on histology or in serum have shown evidence of affecting patient’s outcomes. In terms of genomic profiling, 48% of patients with aggressive variant prostate cancer have mutations in at least two of TP53, RB1, and PTEN.
Interestingly, treatment emergent small cell cancer is seen in “surprisingly large” proportion. This histologically evolves from adenocarcinoma, under selective pressures of treatment. Among these patients who develop t-SCNC (approximately 20%), androgen receptor signalling associated gene expression falls.

Dr. Small then discussed a number of clinical features of t-SCNC, contrasting this with conventional adenocarcinoma. In terms of prognosis, patients with t-SCNC have a dramatically worse prognosis. However, unlike some preconceptions, he noted that visceral metastases are only marginally more common in t-SCNC (44% vs 38%). Similarly, sites of metastases are not particularly predictive of t-SCNC: even among liver biopsies, the vast majority are adenocarcinoma. Similarly, while small cell prostate cancer is associated with low PSA levels, median serum PSA levels were 64.8 in patients with t-SCNC and 46.2 in patients with adenocarcinoma. Further, PSA is almost never completely absent. However, among those with low PSA, t-SCNC is more common but still the minority of cases (approximately 33%). Further, those patients with low PSA secreting cancers are more likely to have RB1 or p53 mutations and respond better to taxanes than with androgen-receptor targeting.
In terms of androgen receptor expression, androgen receptor staining is retained in t-SCNC, unlike in primary small cell prostate cancer. Notably, androgen receptor amplification is seen as commonly in t-SCNC as in adenocarcinoma. However, transcription is repressed.
Moving to demographic characteristics, patients with t-SCNC have similar age, race, and Gleason score distributions as those patients with adenocarcinoma. Further, while serum neuroendocrine markers are only marginally elevated among patients with t-SCNC (resulting in a specificity of 50% and positive predictive value of 22%), sensitivity (95%) and negative predictive value (96%) were high making this a good “rule-out” test.
While there is some shared mutational profile, RB1 and TP53 loss of function variants are more common in t-SCNC (83%) than in adenocarcinoma (34%). Using whole genome sequencing, two hit RB-1 loss was most predictive of impaired survival.
Considering again aggressive variant prostate cancer, Dr. Small emphasized that t-SCNC is a subset of AVPC with poor outcomes. Among the characteristics of AVPC, he noted that visceral metastasis is neither diagnostic for t-SCNC nor does the absence of visceral metastases preclude t-SCNC. Similarly, high PSA expression doesn’t preclude t-SCNC though only 33% of those with low PSA secretion harbour t-SCNC. However, patients with low PSA levels tend to do poorly. He further noted that serum neuroendocrine markers lack specificity for histologically defined t-SCNC. However, whenever possible, p53, RB1, and PTEN should be checked as these have prognostic value.
In terms of therapeutic implications, he highlighted that the NCCN guidelines emphasize chemotherapy and best supportive care as options beginning even in the first line setting. Across a variety of different chemotherapeutic regimes (nearly all with a platinum backbone), he noted “reasonable but short-lived responses”.
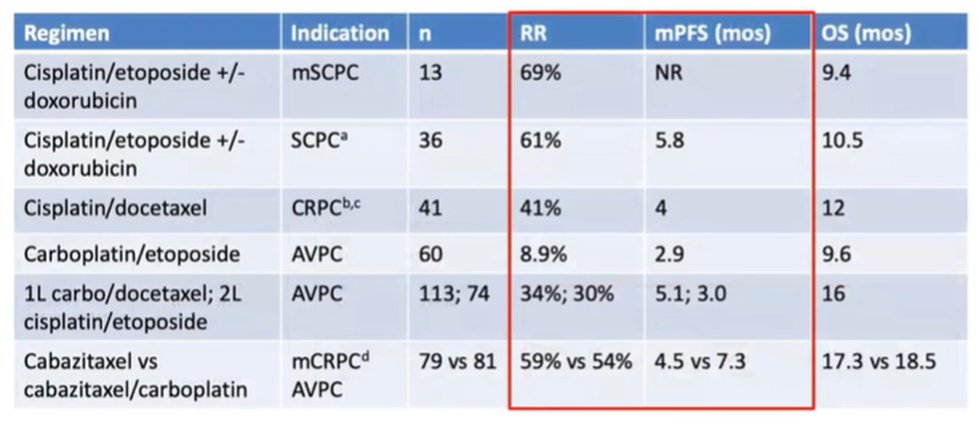
He noted that a randomized phase II trial of cabazitaxel with or without carboplatin showed reasonable responses for both with no significant differences in overall survival. In small cell lung cancer, there is a known benefit of adding immunotherapy, though such an effect is neither known nor approved in prostate cancer.
In closing, Dr. Small highlighted the importance of biopsy where possible as neither disease distribution nor PSA are diagnostic of AVPC or t-SCNC. Platinum-based chemotherapy is warranted, with cabazitaxel and carboplatin showing a modest advantage to cabazitaxel alone. However, whenever possible, clinical trials should be considered for this rare and aggressive subset of prostate cancer.
Presented by: Eric Small, MD, Doris and Donald Fisher Distinguished Professorship in Clinical Cancer Research; Stanford W. Ascherman and Norman R. Ascherman Endowed Chair, UCSF, Professor In Residence of Medicine and Urology; Deputy Director and Chief Scientific Officer; Co-Leader, Prostate Cancer Program, UCSF Helen Diller Family Comprehensive Cancer Center


