(UroToday.com) The 2024 Advanced Prostate Cancer Consensus Conference (APCCC) meeting featured a session on the treatment for biochemical recurrence/PSA persistence, and a presentation by Dr. Barbara Jereczek-Fossa discussing how to select patients with biochemical relapse in whom salvage radiation therapy can be postponed. Dr. Jereczek-Fossa started her presentation by highlighting the intense work that has been done answering the following questions in the biochemical recurrence setting:
- Can we further potentiate ADT?
- What are the benefits of adding first-generation hormone therapy?
- Should all patients benefit from prophylactic pelvic nodal irradiation at biochemical recurrence?
- What imaging exam has the best performance at biochemical recurrence?
- Can we deliver prostate bed radiotherapy hypofractionated regimens?
- Should we escalate the dose of prostate bed radiotherapy?
- What is the optimal timing of prostate bed radiotherapy after radical prostatectomy?
- What are the benefits of prostate bed radiotherapy in cases of biochemical recurrence?

Importantly, there are varying definitions for biochemical recurrence after radical prostatectomy:
- ASCO: Detectable PSA with a subsequent rise
- NCCN: Detectable PSA that increases in >=2 confirmatory tests or increases to PSA levels > 0.1 ng/mL
- AUA/ASTRO/SUO: PSA increase of 0.2 ng/mL and a confirmatory value of >= 0.2 ng/mL
- EAU/EANM/ESUR/ISUP: PSA > 0.4 ng/mL and rising
Thus, there are several definitions of biochemical recurrence, ranging in PSA values from 0.05 – 0.40 ng/mL after surgery. Furthermore, biochemical recurrence after prostatectomy comprises a highly heterogeneous cohort of men:1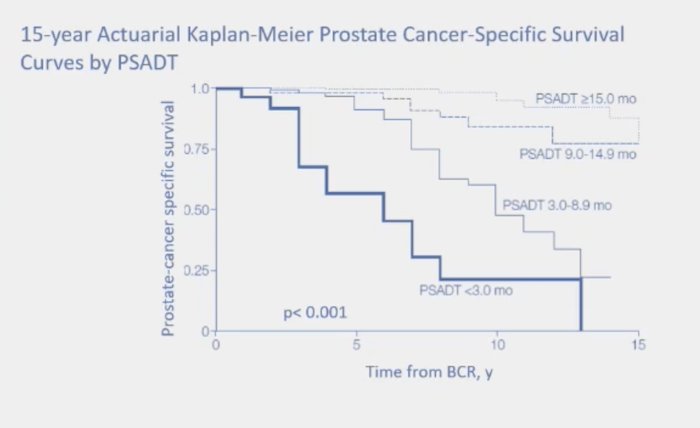
There are a number of prognostic factors associated with biochemical recurrence, including:
- PSA prior to recurrence
- PSA at recurrence
- Time to biochemical recurrence
- pT category
- Positive surgical margins
- Extracapsular extension
- Seminal vesicle invasion
- Lymph node involvement
- PSA doubling time (associated with mortality)
- ISUP grade (associated with mortality)
The EAU has previously established risk categories for biochemical recurrence after radical prostatectomy:
- EAU Low Risk: PSA doubling time > 1 year and pathological ISUP grade < 4
- EAU High Risk: PSA doubling time < 1 year or pathological ISUP grade 4-5
Moreover, these risk groups were recently validated by Preisser et al.2 Among 2,379 patients at 10 centers, there were 805 and 1,574 patients were classified as having EAU low- and high-risk biochemical recurrence, respectively. For both overall survival (p < 0.0001) and cancer-specific survival (p < 0.0001) low-risk biochemical recurrence had improved outcomes versus high-risk: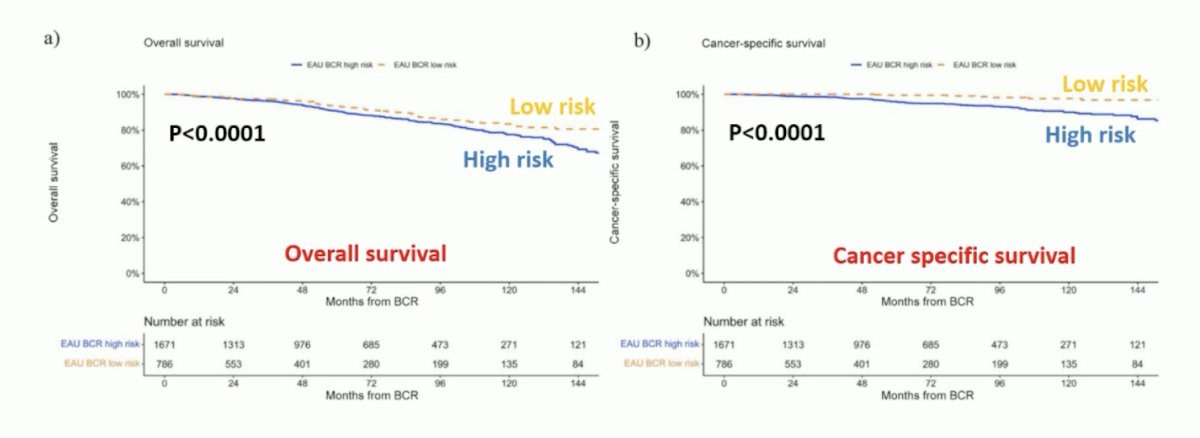
For low-risk biochemical recurrence, 12-year overall survival was 87% versus 78% (p = 0.2) and cancer-specific survival was 100% versus 96% (p = 0.2) for early versus no salvage radiotherapy. For high-risk biochemical recurrence, 12-year overall survival was 81% versus 66% (p < 0.001) and cancer-specific survival was 98% versus 82% (p < 0.001) for early versus no salvage radiotherapy:
In multivariable analyses, early salvage radiotherapy decreased the risk of death (HR 0.55, p < 0.01) and cancer-specific death (HR 0.08, p < 0.001). Late salvage radiotherapy was a predictor of cancer-specific death (HR 0.17, p < 0.01) but not death (HR 0.66, p = 0.1). Dr. Jereczek-Fossa notes that the limitations of the EAU study are that it was retrospective, there was no PSMA PET utilization, and there was no information on ADT utilization. Indeed, in the biochemical recurrence setting there are more and more options as highlighted below: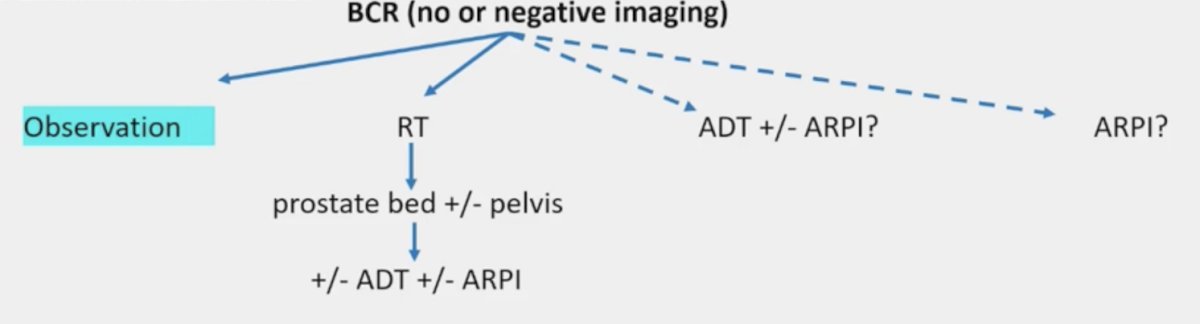
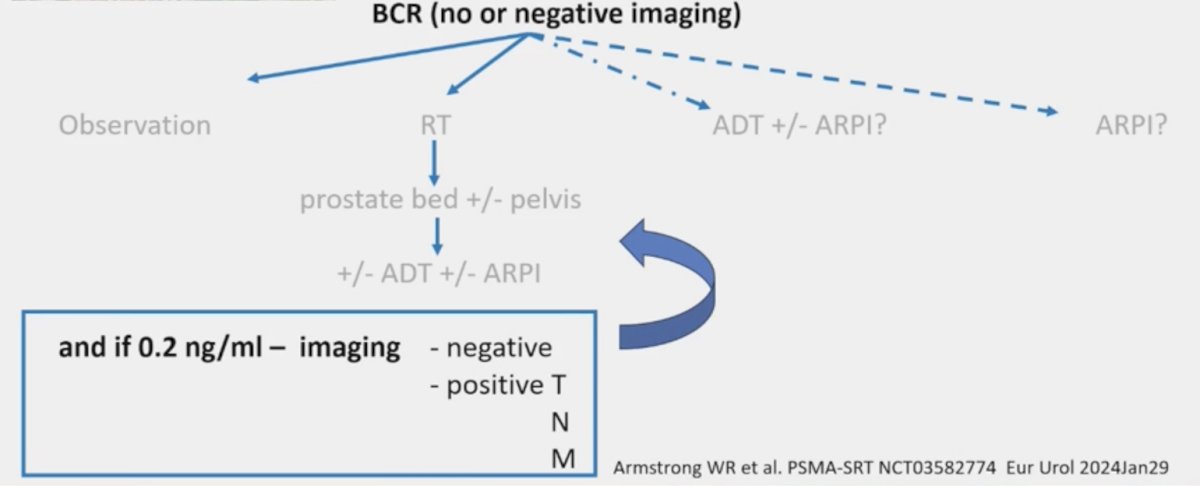
However, in the setting of biochemical recurrence, when is no treatment the best option? Dr. Jereczek-Fossa states that this approach may be most appropriate for those with (i) a short life expectancy, (ii) low-risk biochemical recurrence, (iii) new risk factors delineated by a genomic classifier and/or artificial intelligence, and (iv) favorable imaging. Beyond clinical imaging, genomic testing of the primary tissue includes:
- Oncotype DX
- Prolaris
- ProMark
- Decipher
A recent study from Bologna and colleagues using the PearlDiver Mariner database provided a comprehensive overview of the pivotal genomic tests supporting prostate cancer treatment decisions, analyzing through real-world data trends in their use and the growth of supporting literature evidence.3 Overall, 1,561,203 patients from 2011-2021 with a prostate cancer diagnosis were recorded, of which 20,748 underwent tissue-based genetic testing following diagnosis, representing 1.3% of the total cohort. An increasing trend was observed in the use of all genetic tests. Linear regression analysis showed a statistically significant increase over time in the use of individual tests (all p-values < 0.05). Among the patients who underwent radical prostatectomy, 3,076 received genetic analysis following surgery, representing 1.27% of this group. The following shows the trends in utilization of Decipher and ProMark after radical prostatectomy: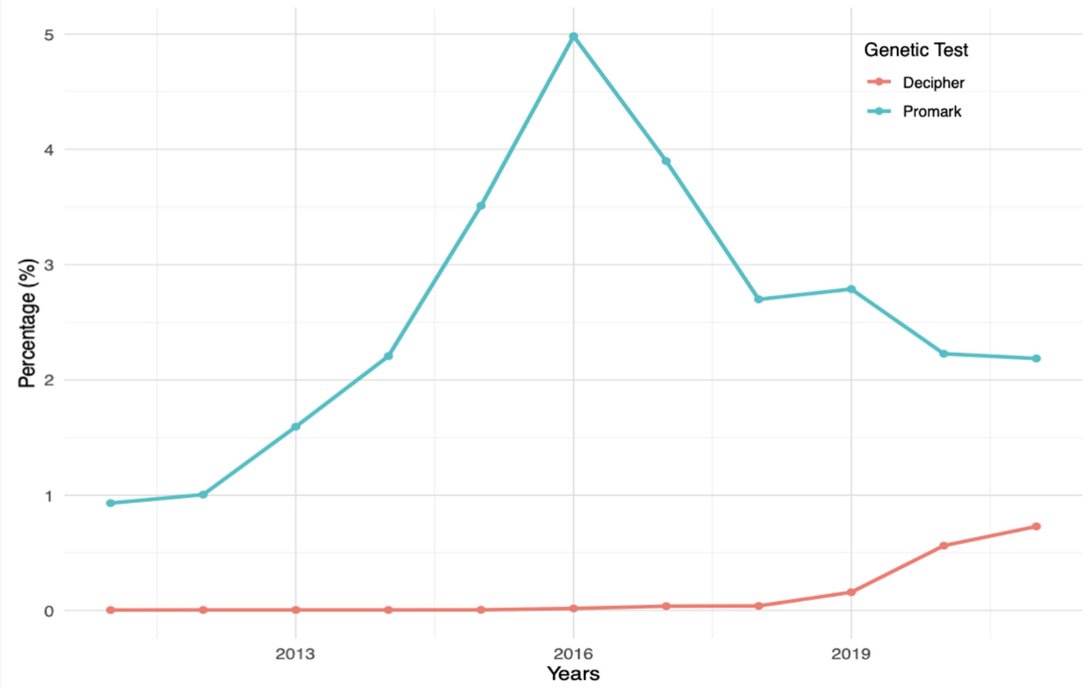
Younger age was associated with a higher probability of testing, with more evidence suggesting testing in the initial diagnosis and choice of adjuvant treatment.
In the SAKK 09/10 trial (Swiss Group for Clinical Cancer Research), Dal Pra et al.4 conducted an analysis of 350 men with biochemical recurrence after radical prostatectomy who received salvage radiotherapy (64 – 70 Gy) without concurrent hormonal therapy or pelvic nodal radiotherapy. The primary endpoint was biochemical progression, whereas secondary endpoints were clinical progression and time to hormone therapy. The analytic cohort included 226 patients, with a median follow-up of 6.3 years. The Decipher genomic classifier score (high versus low-intermediate) was independently associated with the rates of biochemical progression (sHR 2.26, 95% CI 1.42 - 3.60; p < 0.001), clinical progression (HR 2.29, 95% CI 1.32 - 3.98; p = 0.003), and use of hormone therapy (sHR 2.99, 95% CI 1.55 - 5.76; p = 0.001):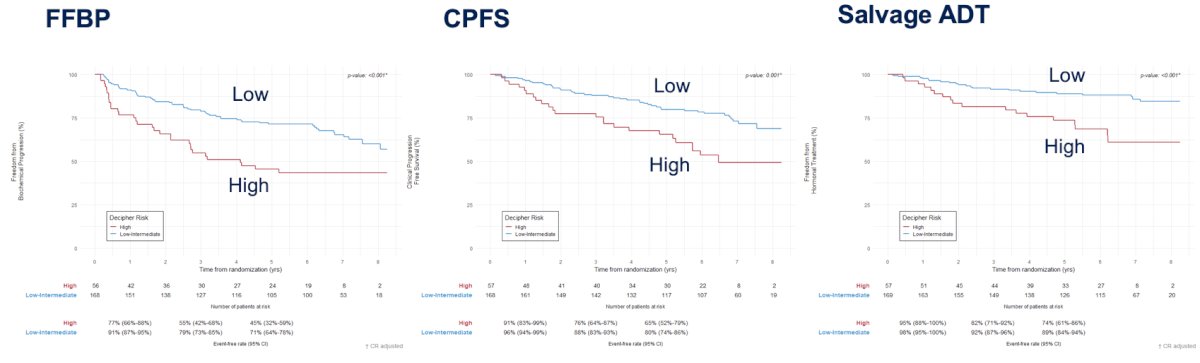
Dr. Jereczek-Fossa notes that with regards to genomic testing, for patients with a high risk (> 0.6) Decipher score after radical prostatectomy, patients may benefit from radiotherapy with concurrent ADT, and may benefit from earlier, more intense multimodality therapy. Currently, both the NCCN and AUA/ASTRO/SUO guidelines recommend genomic testing to define a patient’s overall risk profile for recurrence.
On the theme of beyond classical risk factors in the setting of biochemical recurrence, Dr. Jereczek-Fossa discussed artificial intelligence and deep learning – a technique in which computer software learns patterns from multiple examples. Previously, Pinckaers et al.5 utilized artificial intelligence to predict biochemical recurrence among 182 and 204 patients from two institutions: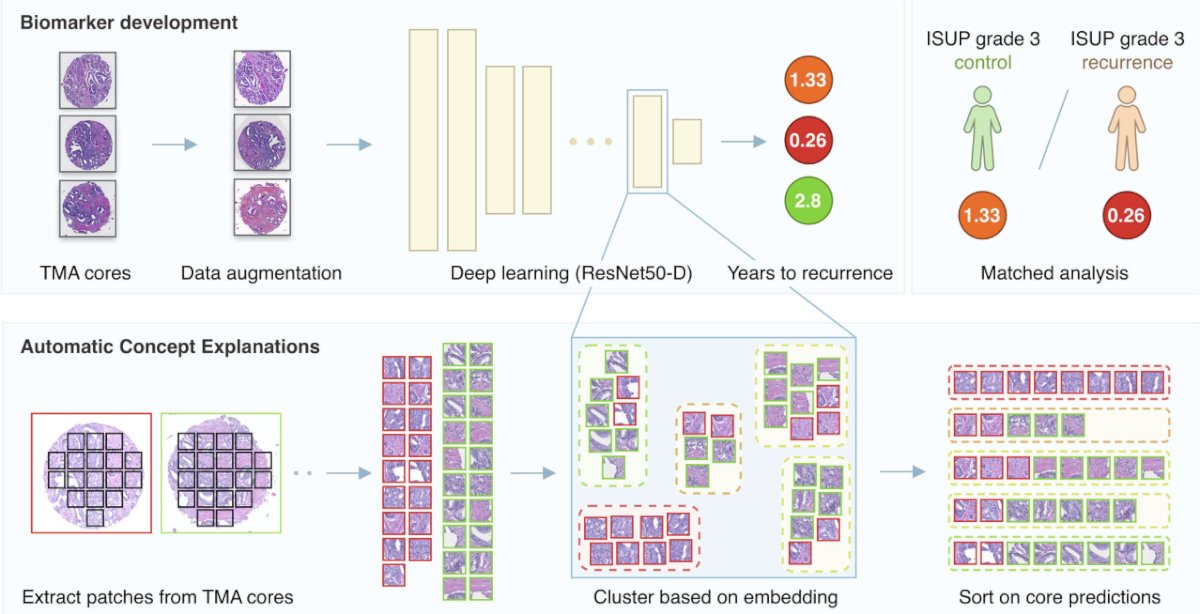
They found that visual markers were associated with biochemical recurrence, including cribriform-like growth patterns, also providing a continuous score for predicting the time to biochemical recurrence.
Dr. Jereczek-Fossa emphasized some of the potential pitfalls of postponing salvage radiotherapy, which may include (i) more frequent follow-up, (ii) anxiety, and (iii) losing the window of opportunity of a highly efficacious single treatment. Salvage radiotherapy at a very low PSA has a very high probability of disease control, whereas a salvage treatment at a higher PSA leads to longer time on treatment and higher cost of treatment.
Currently, there is no consensus for monitoring in EAU low-risk biochemical recurrence with the EAU guidelines suggesting to offer monitoring, including PSA, to EAU low-risk biochemical recurrence patients (Strength rating: Weak). There is strong EAU guideline evidence for salvage radiotherapy if the PSMA PET is negative and not waiting for a threshold PSA if salvage radiotherapy is indicated:
- A negative PSMA PET/CT scan should not delay salvage radiotherapy if otherwise indicated (Strength rating: Strong)
- Do not wait for a PSA threshold before starting treatment. Once the decision for salvage radiotherapy has been made, salvage radiotherapy (at least 64 Gy) should be given as soon as possible (Strength rating: Strong)
There are currently 100 trials ongoing in this disease space. Dr. Jereczek-Fossa highlighted the DIPPER trial, which is randomizing men with EAU low-risk biochemical recurrence to surveillance versus radiotherapy to the prostate bed +/- pelvic lymph nodes. The primary endpoint is event-free survival at 3 years:
Dr. Jereczek-Fossa concluded her presentation discussing how to select patients with biochemical relapse in whom salvage radiation therapy can be postponed with the following take-home messages:
- Biochemical recurrence is a very heterogeneous disease space
- EAU risk categories may be helpful in patient stratification selected low-risk patients may not need salvage radiotherapy
- Not all low-risk patients are the same
- Biochemical recurrence is a unique opportunity to impact survival and delay metastasis and there may be a risk of losing the window of opportunity
- Advances in molecular and genetic profiling of the tumor with clinical risk assessments provide deeper insights into disease aggressiveness
- Risk-adapted and image-based salvage treatment is the future: genomic classifiers and artificial intelligence
Presented by: Barbara A. Jereczek-Fossa, MD, PhD, European Institute of Oncology IRCCS, Milan, Italy
Written by: Zachary Klaassen, MD, MSc - Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Advanced Prostate Cancer Consensus Conference (APCCC) Meeting, Lugano, Switzerland, Thurs, Apr 25 - Sat, Apr 27, 2024.
References:
- Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005 Jul 27;294(4):433-439.
- Preisser F, Abrams-Pompe RS, Stelwagen PJ et al. European Association of Urology Biochemical Recurrence Risk Classification as a Decision Tool for Salvage Radiotherapy—A Multicenter Study. Eur Urol. 2024;85:164-170.
- Bologna E, Ditonno F, Licari LC, et al. Tissue-based genomic testing in Prostate Cancer: 10-year analysis of National Trends on the use of Prolaris, Decipher, ProMark, and Oncotype DX. Clin Pract. 2024 Mar 19;14(2):508-520.
- Dal Pra A, Ghadjar P, Hayoz S, et al. Validation of the Decipher genomic classifier in patients receiving salvage radiotherapy without hormone therapy after radical prostatectomy - an ancillary study of the SAKK 09/10 randomized clinical trial. Ann Oncol. 2022;33(9):950-958.
- Pinckaers H, van Ipenburg J, Melamed J, et al. Predicting biochemical recurrence of prostate cancer with artificial intelligence. Commun Med (Lond). 2022 Jun 8;2:64.


