(UroToday.com) The 2024 Advanced Prostate Cancer Consensus Conference (APCCC) meeting featured a session on the management of metastatic hormone-sensitive prostate cancer (mHSPC), and a presentation by Dr. Robert Jones discussing the patients with synchronous low-volume mHSPC that should receive combination systemic therapy + local treatment of the primary, and the patients in which ADT alone + radiotherapy is sufficient treatment. Dr. Jones started by presenting a case of one of his patients. This patient was born in 1930 and in 2001 was diagnosed with T2N0M1 “low volume” prostate cancer and treated with flutamide. In 2007, he had PSA failure and was treated with goserelin, and in 2012 bicalutamide was added to his regimen. In 2016, abiraterone was added, and in 2020, at the age of 90 years of age, he died with negative scans, with an unspecified cause of death. Dr. Jones notes that fatigue, sarcopenia, and osteopenia dominated the final years of his life. Reflecting back on this case, in 2022 Dr. Jones notes that there were 3 points of good news. First, the prognosis of low volume mHSPC is (relatively) good. In the STAMPEDE low-volume mHSPC patients, median overall survival is 63.6 months:1
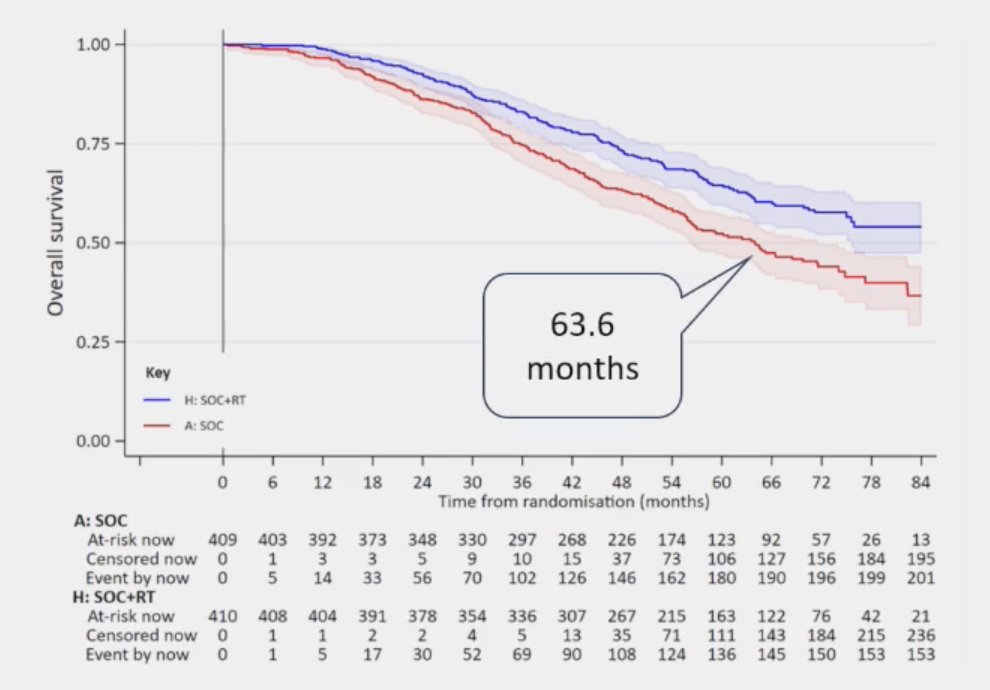
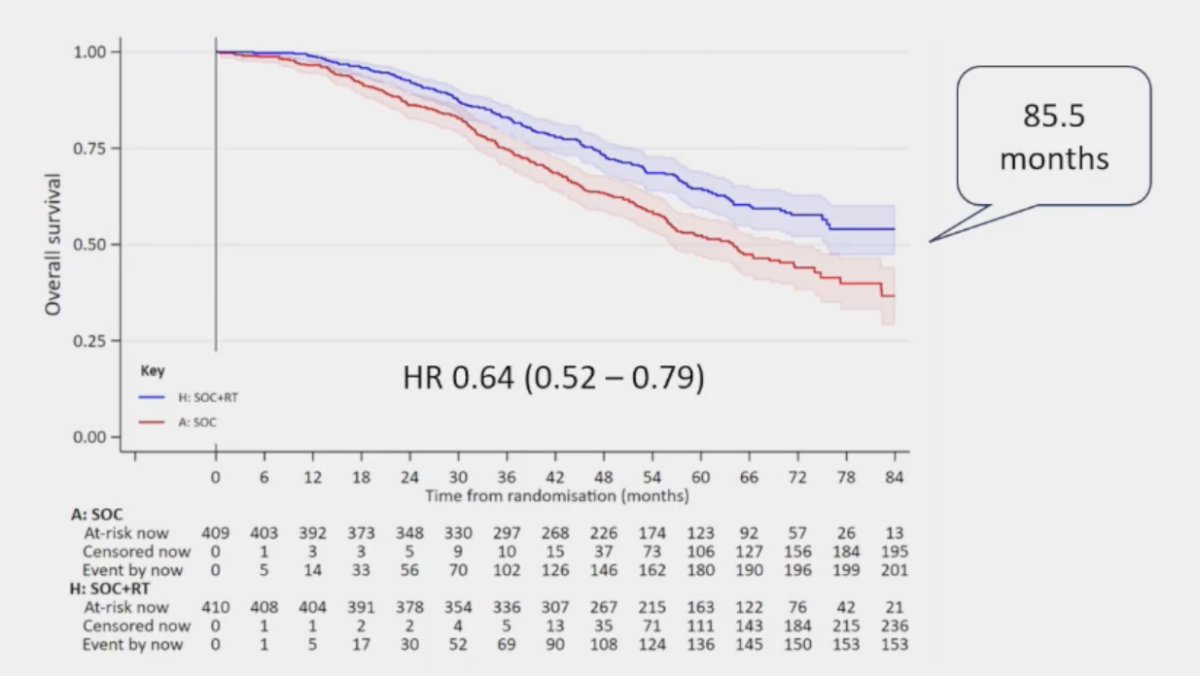
Secondly, radiotherapy improves overall survival in STAMPEDE low-burden mHSPC, with a median overall survival of 85.5 months (HR 0.64, 95% CI 0.52 – 0.79):1
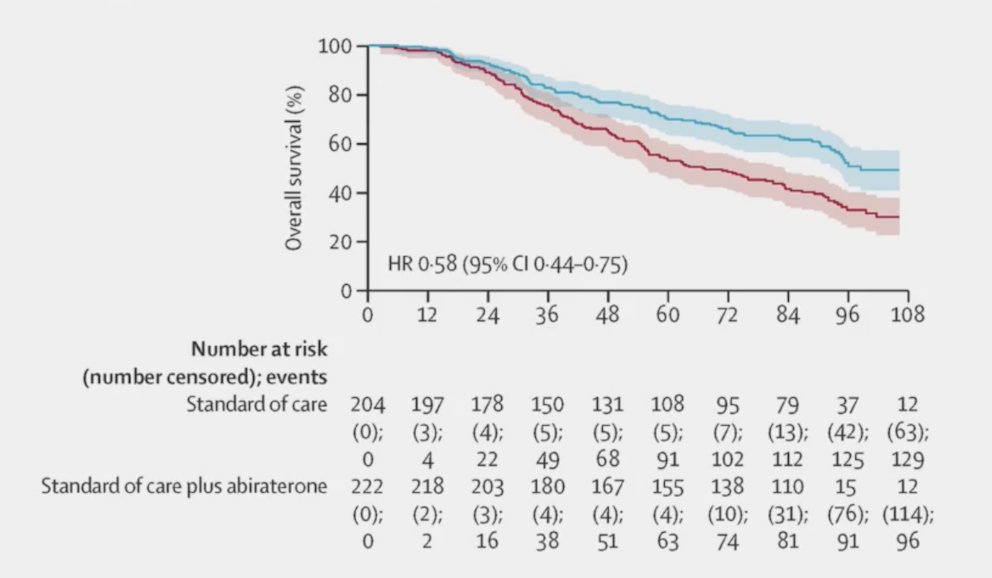
Thirdly, ARPIs improve overall survival (HR 0.58, 95% CI 0.44-0.75),2 with the following highlighting the impact of abiraterone in the STAMPEDE platform:
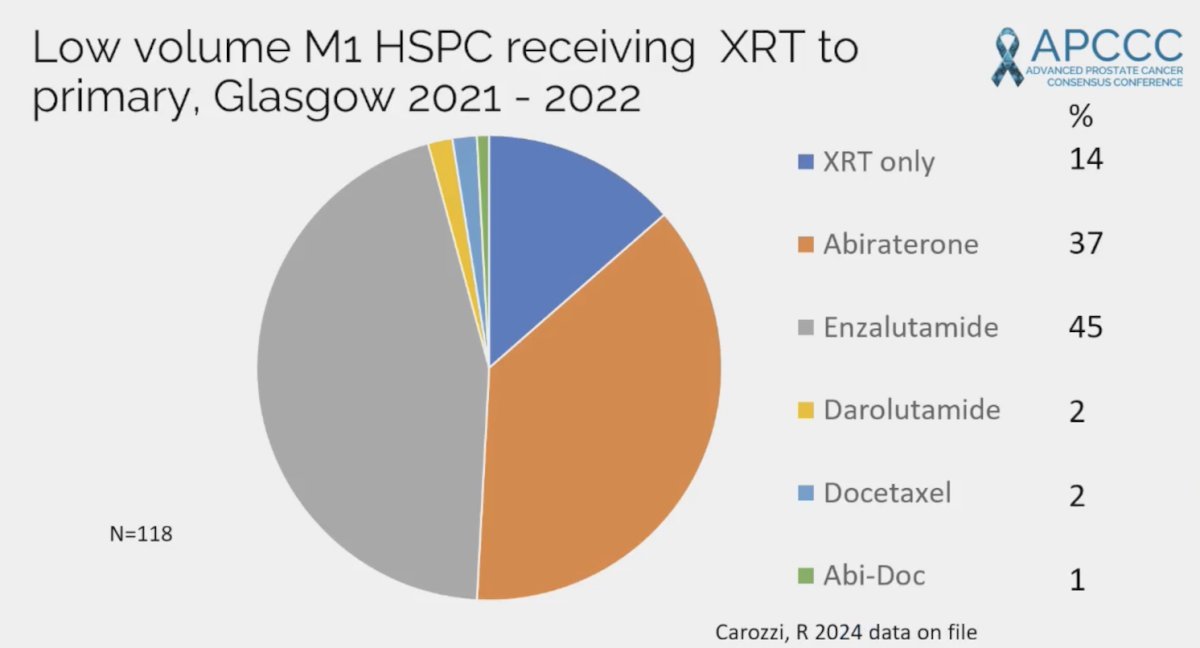
Dr. Jones then discussed the treatment patterns for low volume M1 HSPC receiving radiotherapy to the primary at his institution in Glasgow from 2021-2022. This included 86% of patients getting radiotherapy + treatment intensification, most commonly with enzalutamide (45%):
The most relevant data with regards to what’s new since 2022, is the PEACE-1 data, presented at ASCO 2023. The trial design for PEACE-1 is as follows:
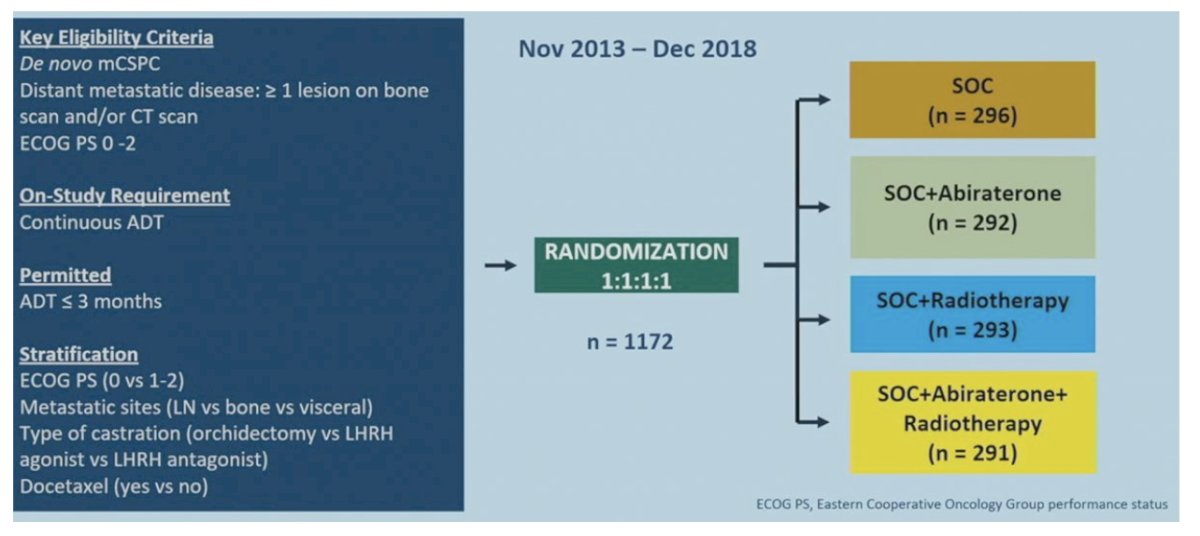
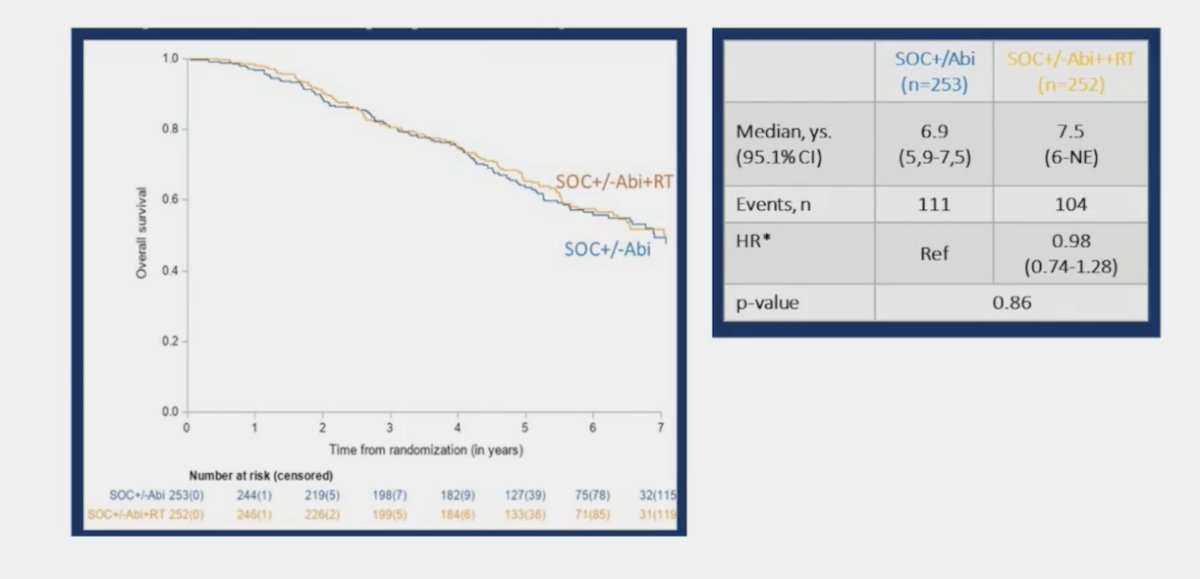
Overall survival in the low volume population assessing standard of care +/- abiraterone versus standard of care +/- abiraterone + radiotherapy showed no survival benefit with the addition of radiotherapy (HR 0.98, 95% CI 0.74-1.28):
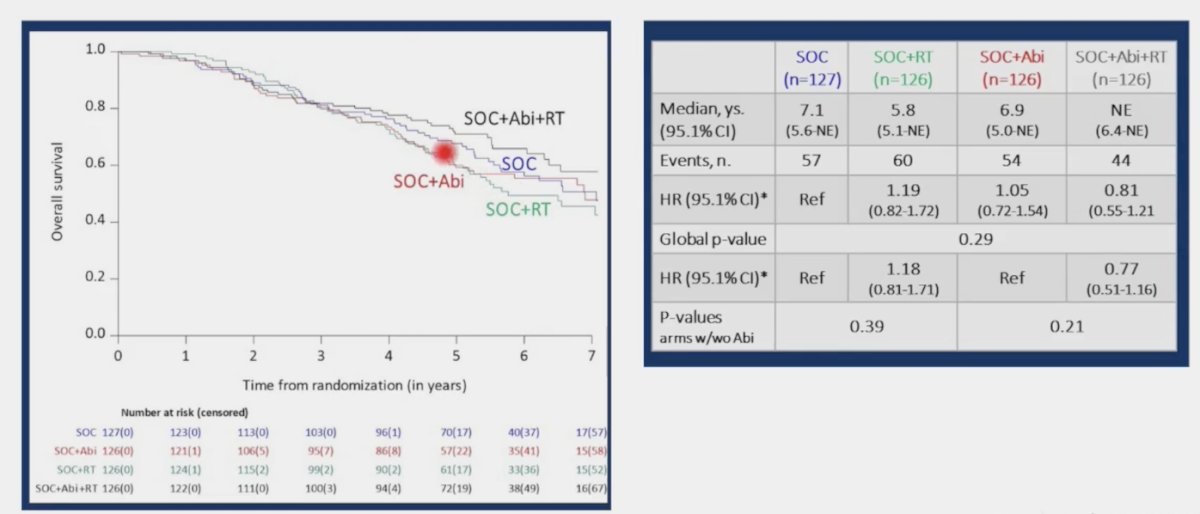
In the standard of care + abiraterone arms, the addition of prostate radiotherapy was associated modest, non-significant overall survival benefits (HR 0.77, 95% CI 0.51 – 1.16, p = 0.21). Similarly, the addition of prostate radiotherapy to standard of care alone did not improve overall survival (HR 1.18, 95% CI 0.81- 1.71, p = 0.39).
In a recently published network meta-analysis assessing prostate radiotherapy in low-volume mHSPC patients, Roy and colleagues3 found that in a population with de novo mHSPC and no docetaxel use, there was insufficient evidence of a difference in overall survival between standard of care + ARPI + radiotherapy versus standard of care + ARPI (HR 0.76, 95% CI 0.51-1.16) and standard of care + radiotherapy versus standard of care + ARPI (HR 1.10, 95% CI: 0.92-1.42).
Dr. Jones also notes that radiotherapy toxicity to the primary is minimal, based on data from STAMPEDE and PEACE 1:
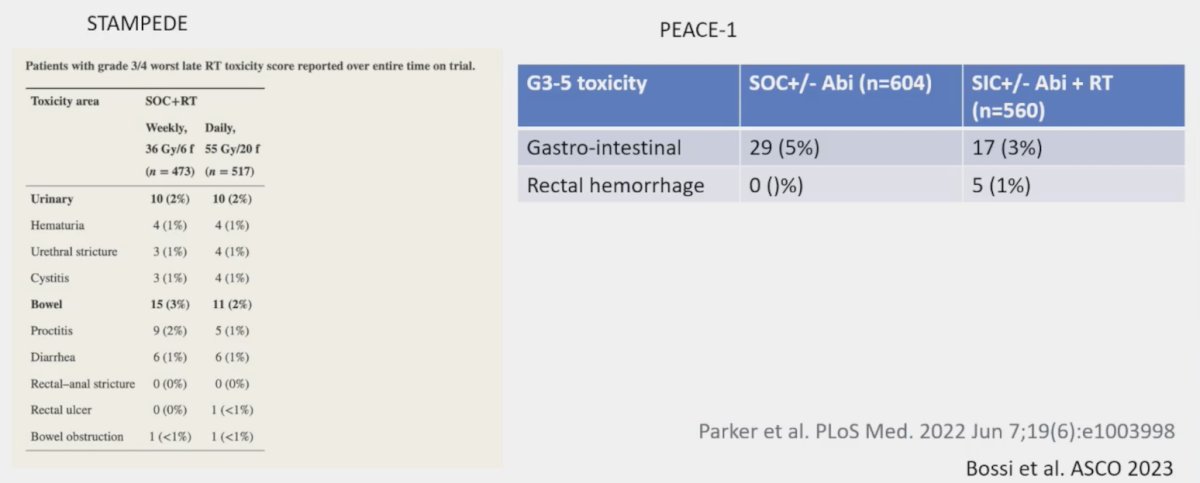
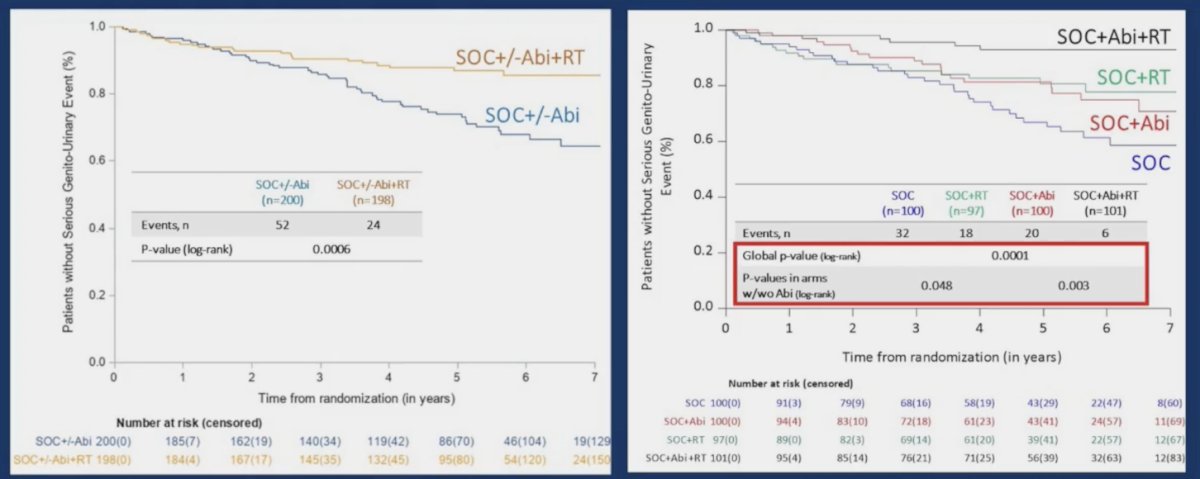
Interestingly, the addition of prostate radiotherapy to standard of care +/- abiraterone in the low-volume cohort was associated with significant improvements in the time to serious genitourinary events (p = 0.0006). This overall benefit was consistent irrespective of whether patients had prostate radiotherapy added to standard of care + abiraterone (p = 0.003) or standard of care alone (p = 0.048):
Dr. Jones concluded his presentation discussing the patients with synchronous low-volume mHSPC that should receive combination systemic therapy + local treatment of the primary, and the patients in which ADT alone + radiotherapy is sufficient treatment with the following take-home messages:
- The value of triplet radiotherapy + ARPI + ADT is uncertain
- Radiotherapy does not appear to add value to ADT + ARPI
- We need to consider the relative contraindications to ARPIs
- We need to consider life expectancy, risk of metastatic progression versus risk of local morbidity
- However, most of those patients getting radiotherapy will also get an ARPI
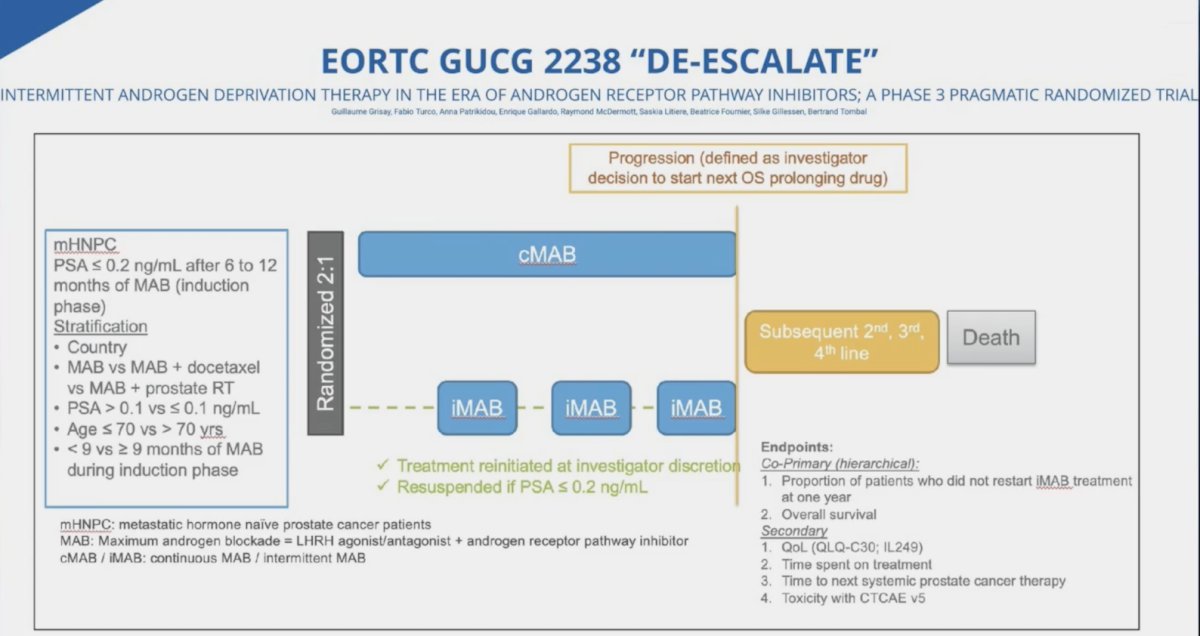
Finally, Dr. Jones mentioned the DE-ESCALATE EORTC GUCG 2238 trial, which will assess intermittent androgen deprivation therapy in the era of androgen receptor pathway inhibitors in a phase 3 setting:
Presented by: Robert Jones, MD, PhD, Cardiff University and Velindre University National Health Service Trust, Cardiff, UK
Written by: Zachary Klaassen, MD, MSc - Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Advanced Prostate Cancer Consensus Conference (APCCC) Meeting, Lugano, Switzerland, Thurs, Apr 25 - Sat, Apr 27, 2024.
References:
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the prostate for men with metastatic prostate cancer in the UK and Switzerland: Long-term results from the STAMPEDE randomised controlled trial. PLoS Medicine. 2022;19(6):e1003998.
- Attard G, Murphy L, Clarke NW, et al. Abiraterone acetate plus prednisolone with or without enzalutamide for patients with metastatic prostate cancer starting androgen deprivation therapy: Final results from two randomized phase 3 trials of the STAMPEDE platform protocol. Lancet Oncol. 2023 May;24(5):443-456.
- Roy S, Fervaha G, Spratt DE, et al. Prostate Radiotherapy in Low-volume Metastatic Hormone-sensitive prostate cancer: A network meta-analysis. Eur Urol. 2024 Apr 2 [Epub ahead of print].
Related Content:


