(UroToday.com) The 2024 Advanced Prostate Cancer Consensus Conference (APCCC) held in Lugano, Switzerland was host to a high-risk and locally advanced prostate cancer session. Dr. Daniel Spratt discussed the use of genomic classifiers and artificial intelligence tools as predictors of treatment benefit in prostate cancer patients.
Dr. Spratt noted that there are significant limitations to current risk stratification tools that often rely on conventional imaging findings and an ‘outdated’ histologic parameter, Gleason Score, to dictate treatment decision-making. While Gleason Score is routinely used as one of the primary tools to risk stratify patients in practice, Dr. Spratt noted that this grade classification system was initially described as independent of oncologic outcomes and was then subsequently correlated to only 7 prostate cancer mortality events, as illustrated in the table below.
Indeed, the underwhelming predictive performance of Gleason Score for guiding treatment was recently confirmed in an individual patient data meta-analysis of 10 radiotherapy-related RCTs of 10,535 patients. In this meta-analysis, the investigators demonstrated that patient Gleason Score has limited value for predicting which patients would benefit from the addition of short-term or long-term ADT to radiotherapy for metastasis-free or overall survival.1 As such, Dr. Spratt argued that the revised Gleason scoring system was not designed with the intent of optimal contemporary prognostication or prediction of treatment response.
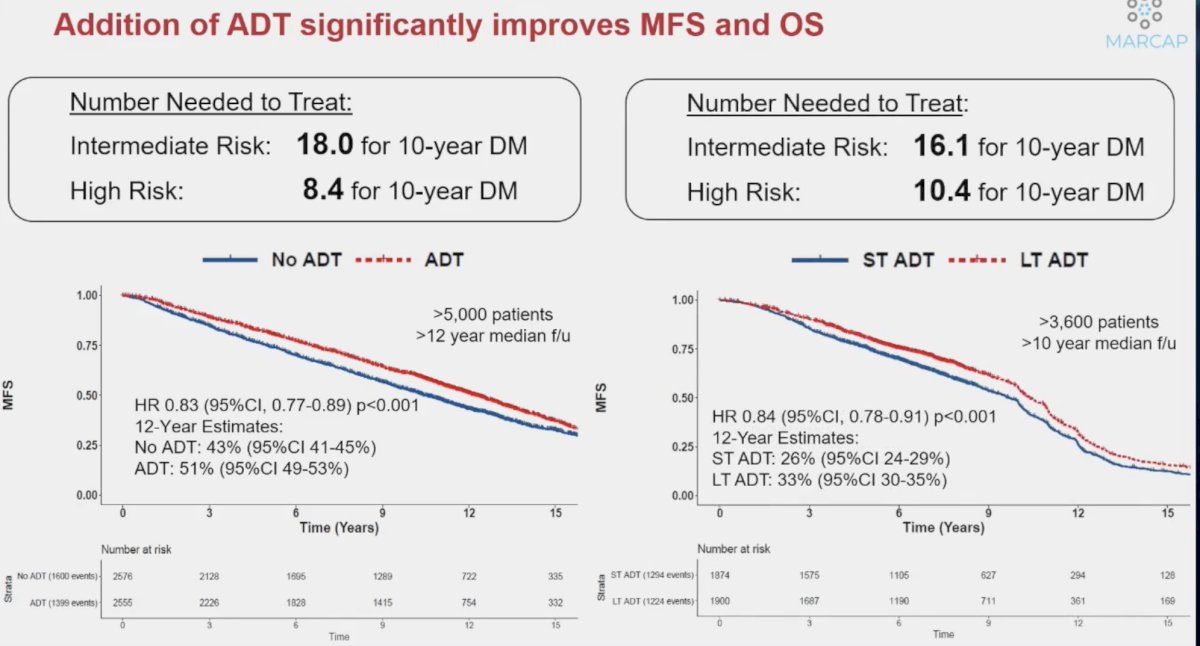
While the addition of ADT to radiotherapy significantly improves metastasis-free and overall survival, the numbers needed to treat to prevent one distant metastasis event remain unacceptably high, ranging between 8 and 18. As such, we need an improved personalized approach to maximize benefit, while minimizing toxicity.
What are the most recent advances in the personalization of therapy for prostate cancer patients? The majority of current and ongoing research in this field has focused on systemic therapy intensification for intermediate risk patients planned for radiotherapy. The 22-gene genomic classifier (Decipher®) has been assigned a Simon level of evidence 1B by the most recent NCCN guidelines as a prognostic biomarker for metastatic risk. This biomarker has important treatment implications when considering the addition of short-term ADT to radiotherapy for patients with intermediate-risk disease. Currently, the NCCN panel suggests that radiotherapy alone should be considered for patients with a low Decipher® score and NCCN intermediate-risk disease. Conversely, the addition of short-term ADT can be considered for patients with a high Decipher® score given their increased risk of distant metastases.
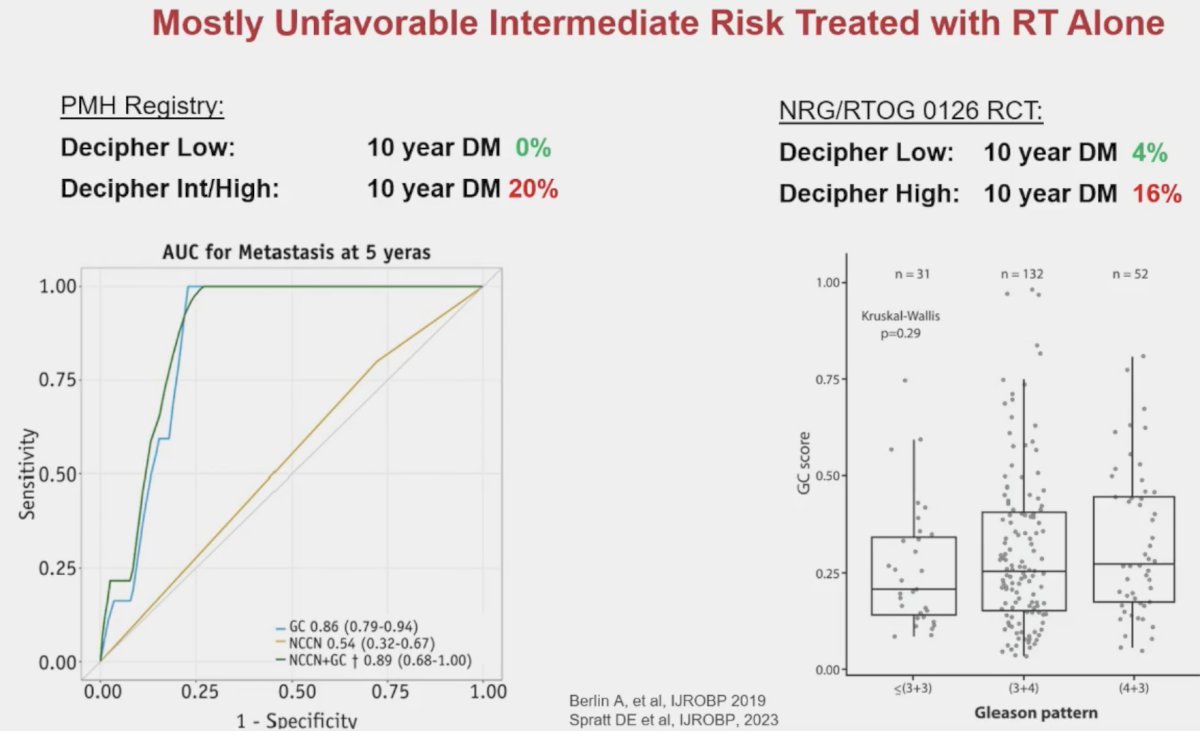
This biomarker has been demonstrated in multiple series to have important prognostic value for the risk stratification of patients with intermediate-risk disease. In 2019, Berlin et al. demonstrated that intermediate-risk patients with low Decipher® scores do not experience distant metastases within 10 years. Conversely, those with Decipher® intermediate/high scores have a 10-year rate of distant metastases of 20%. Significantly, Decipher® score had an area under the curve (AUC) of 0.86 for predicting 5-year metastases, significantly outperforming the NCCN risk classification that had an AUC of only 0.54.2
Similarly, using data from NRG Oncology/RTOG 0126, a randomized phase 3 trial of men with intermediate-risk prostate cancer randomized to 70.2 Gy versus 79.2 Gy of radiation therapy without ADT, Spratt et al. demonstrated that intermediate risk patients with low Decipher® scores had a 10-year distant metastases rate of only 4%, compared to 16% for those with Decipher® high scores.3
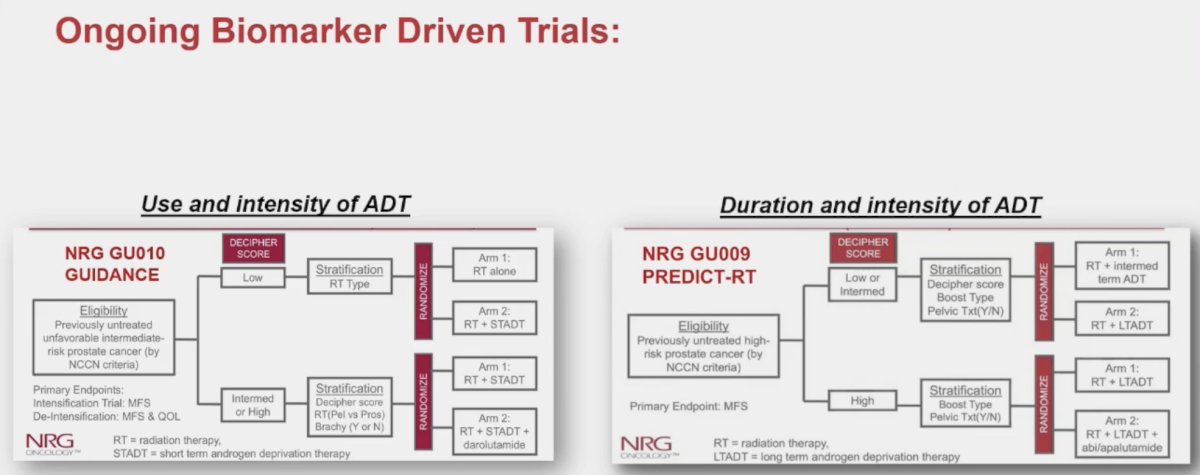
The Decipher® 22-gene genomic classifier is currently being evaluated across numerous prostate cancer settings, including localized disease, post-operative and recurrent settings, mHSPC, and non-metastatic CRPC disease states:

Another exciting, emerging tool is multi-modal artificial intelligence (MMAI). This is the first biomarker to demonstrate predictive value for the treatment of prostate cancer patients. This has been recently acknowledged by the updated NCCN guidelines which have assigned this biomarker a Simon level of evidence 1B for both predictive and prognostic purposes. MMAI has predictive value for adding short-term ADT to radiotherapy for intermediate-risk patients, stating the following: “Patients with intermediate-risk prostate cancer planning to receive radiotherapy, those with biomarker positive disease, and especially those with unfavorable intermediate risk disease, should be recommended for the addition of short-term ADT regardless of radiotherapy dose or type. notwithstanding contraindications to ADT. Those with biomarker (-) tumors, especially tumors with more favorable prognostic risk, may consider the use of radiotherapy alone.”
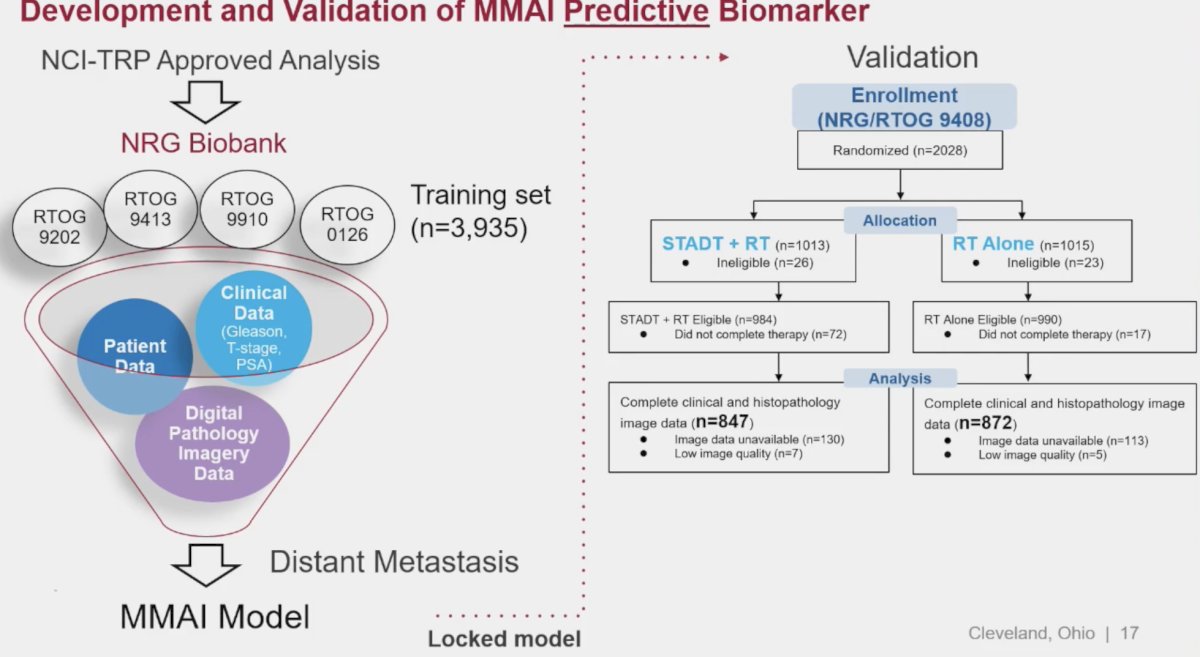
The evidence to support this recommendation originates from the pivotal study by Spratt et al. published in NEJM Evidence in 2023. In this study, the investigators used digital pathology images from pretreatment prostate tissue and clinical data from 5,727 patients enrolled in five phase 3 randomized trials (RTOG 9202, 9413, 9910, 0126, and 9408), in which treatment was radiotherapy +/- ADT, as their data source to develop and validate an artificial intelligence (AI)–derived predictive patient-specific model that would determine which patients would develop the primary end point of distant metastasis. The model used baseline data to provide a binary output that a given patient will likely benefit from ADT or not. After the model was locked, validation was performed using data from NRG Oncology/RTOG 9408 (n=1594), a trial that randomly assigned men to radiotherapy +/- 4 months of ADT.
Overall, in the NRG/RTOG 9408 validation cohort (14.9 years of median follow-up), ADT significantly improved time to distant metastasis. Of these enrolled patients, 34% were model positive, and ADT significantly reduced the risk of distant metastasis compared with radiotherapy alone. Of 1,051 patients who were model negative, ADT did not provide benefit.3
Next, Dr. Spratt discussed the prognostic value of Decipher® for patients with NCCN high-risk disease, specifically with regards to the decision to add short-term versus long-term ADT to radiotherapy. The NCCN panel states that: “Radiotherapy + long-term ADT should be recommended for most patients with NCCN high-risk disease regardless of the genomic classifier score outside of a clinical trial, even with dose-escalated radiotherapy or brachytherapy boost. However, patients with a genomic classifier low risk score should be counseled that the absolute benefit of long-term ADT over short-term ADT is smaller than for patients with genomic classifier high risk scores and when accounting for patient age, comorbidities, and patient preferences, it may be reasonable with shared decision-making to use a duration shorter than long-term ADT.”
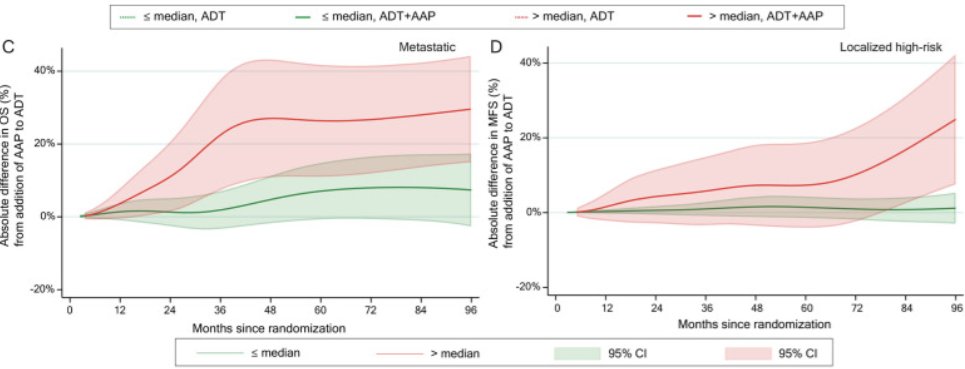
Decipher® has recently been evaluated in a post-hoc analysis of the STAMPEDE abiraterone +/- enzalutamide systemic therapy intensification pooled data for high/very high-risk patients receiving radiotherapy + ADT. This demonstrated that there was minimal benefit to the addition of an androgen receptor pathway inhibitor (ARPI) to radiotherapy + ADT for those with a low Decipher® score, irrespective of whether they had localized, high-risk or metastatic disease, whereas those with a Decipher® high score derived a significant benefit.5
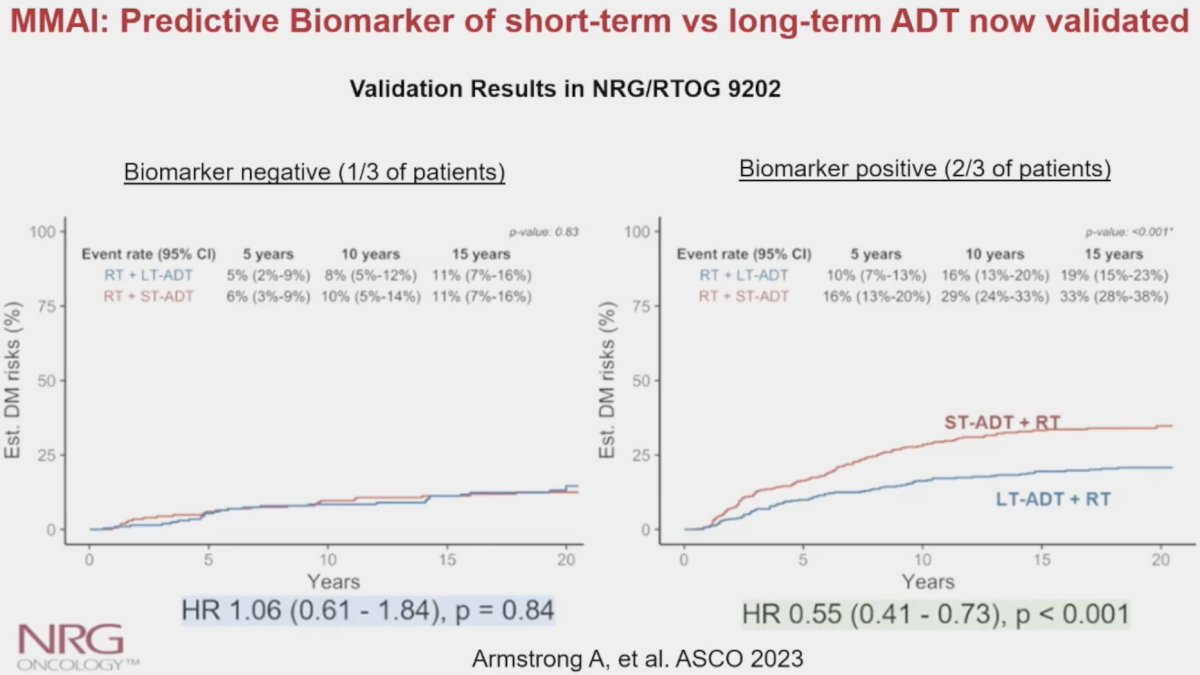
In a recently presented study at ASCO 2023 using data from the NRG Oncology/RTOG 9202 trial (80% high-risk patients), Armstrong et al. demonstrated that the MMAI biomarker (ArteraAI) was a significant predictor of the utility of long-term ADT use in this cohort (interaction p=0.04). As demonstrated below, patients in the MMAI biomarker negative group (n=407) derived no benefit from long-term ADT use, compared to short-term ADT use (HR: 1.06, 95% CI: 0.61 – 1.84, p=0.84). Conversely, patients in the MMAI biomarker positive group had significant improvements in the distant metastasis rates with long-term ADT (HR: 0.55, 95% CI: 0.41 – 0.73, p<0.001). Accordingly, approximately 1/3 of men with high-risk prostate cancer could have safely avoided long-term ADT based on the results of this predictive biomarker analysis.
Dr. Spratt noted that there are currently numerous ongoing biomarker-driven trial evaluating the use, duration, and intensity of ADT for patients planned for radiotherapy:
Dr. Spratt concluded his presentation by highlighting why personalization of ADT/ARPI therapy is critical in patients with high-risk, node-positive M0 disease:
- Modern high-risk patients have, on average, excellent oncologic outcomes with radiotherapy + 2 years of ADT:
- 5-year biochemical recurrence of only ~ 5-8% (FLAME randomized trial, POP-RT randomized trial, etc)
- Thus, the bar to accept quality of life declines and toxicity should be very high
- ADT prolongation (i.e., long-term versus short-term ADT) significantly increases:
- Cardio/cerebrovascular disease (DART phase III trial)
- Other-cause mortality (MARCAP)
- There should be caution in use of ARPIs in patients with M0 disease, especially those >70 years old:
- Await ATLAS, ENZARAD, DASL-HiCAP
Presented by: Daniel Spratt, MD, Vincent Smith Professor and Chair of Radiation Oncology, University Hospitals, Seidman Cancer Center, Case Western Reserve University SOM, Cleveland, OH
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 Advanced Prostate Cancer Consensus Conference (APCCC) Meeting, Lugano, Switzerland, Thurs, Apr 25 - Sat, Apr 27, 2024.
References:
- Dess RT, Suny Y, Bolla M, et al. Prognostic and Predictive Performance of Routine Clinicopathologic Variables in 10,535 Men Enrolled on Randomized Phase III Trials in Localized Prostate Cancer. Int J Radiat Oncol Biol Phys. 2021;111(3):S78.
- Berlin A, Murgic J, Hosni A, et al. Genomic Classifier for Guiding Treatment of Intermediate-Risk Prostate Cancers to Dose-Escalated Image Guided Radiation Therapy Without Hormone Therapy. Int J Radiat Oncol Biol Phys. 2019;103(1): 84-91.
- Spratt DE, Liu VYT, Michalski J, et al. Genomic Classifier Performance in Intermediate-Risk Prostate Cancer: Results From NRG Oncology/RTOG 0126 Randomized Phase 3 Trial. Int J Radiat Oncol Biol Phys. 2023;117(2): 370-7.
- Spratt DE, Tang S, Sun Y, et al. Artificial Intelligence Predictive Model for Hormone Therapy Use in Prostate Cancer. NEJM Evidence. 2023;2(8).
- Parry MA, Grist E, Mendes L, et al. Clinical testing of transcriptome-wide expression profiles in high-risk localized and metastatic prostate cancer starting androgen deprivation therapy: an ancillary study of the STAMPEDE abiraterone Phase 3 trial. Res Sq. 2023.
Related Content: