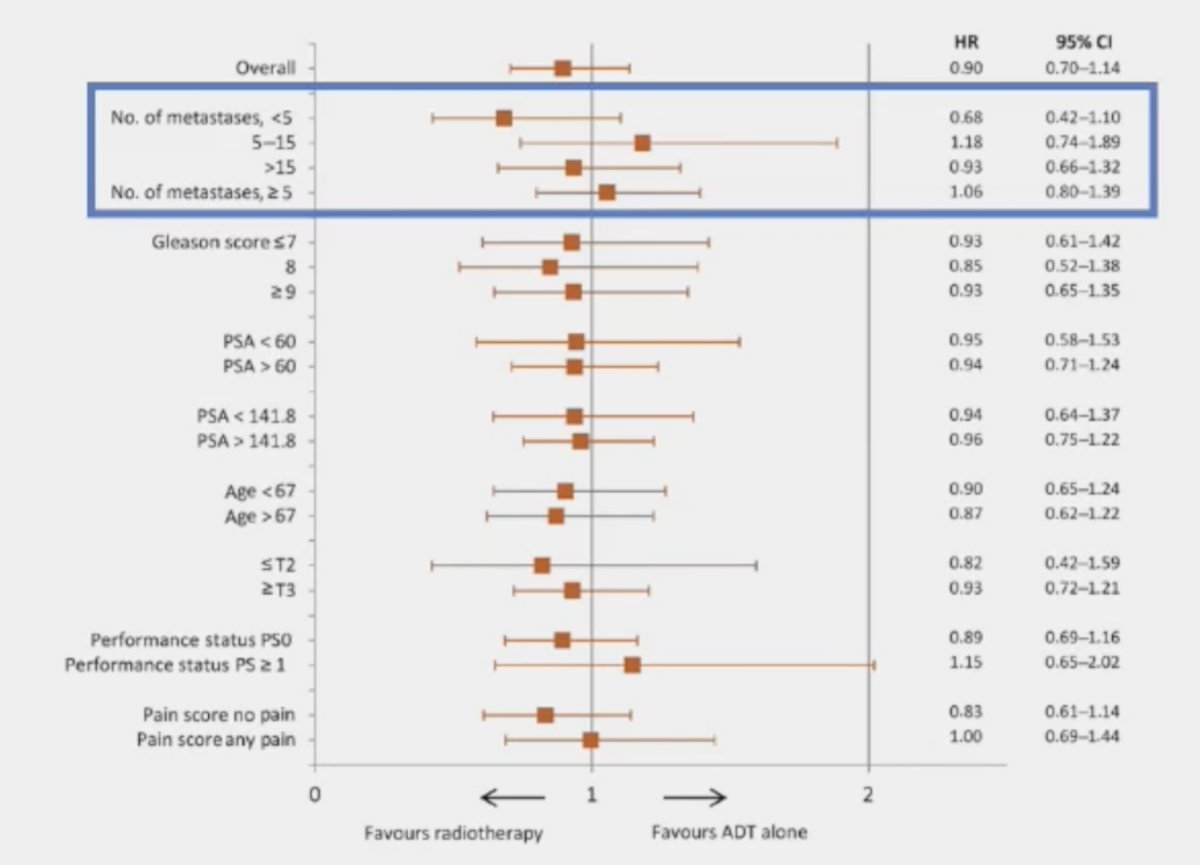
(UroToday.com) The 2024 Advanced Prostate Cancer Consensus Conference (APCCC) meeting featured a session on the management of metastatic hormone sensitive prostate cancer (mHSPC), and a presentation by Dr. Sandy Srinivas discussing whether the primary should be treated in synchronous high-volume mHSPC. Dr. Srinivas started her presentation by discussing the HORRAD trial, which was a multicenter prospective trial of 432 patients with previously untreated, de novo mHSPC at 28 centers across The Netherlands between November 2004 and September 2014.1 All eligible patients had a PSA >20 ng/ml and documented bone metastases on bone scan. Patients were randomized 1:1 to either androgen suppression alone or with external beam radiotherapy. The median PSA level was 142 ng/ml. After a median follow up of 47 months, the primary outcome of median overall survival was non-significantly different: 45 months in the radiotherapy/ADT arm versus 43 months in the ADT alone arm (HR: 0.90, 95% CI: 0.70 - 1.14, p = 0.40). Subgroup analysis by number of metastatic lesions suggested a potential overall survival benefit for radiotherapy in patients with <5 metastatic sites (HR: 0.68, 95% CI: 0.42 - 1.10):
Dr. Srinivas then discussed STAMPEDE Arm H, an open label, randomized controlled phase III trial of 2,061 men at 117 hospitals across Switzerland and the UK.2 This arm randomized patients with de novo mHSPC in a 1:1 fashion to standard of care with or without radiotherapy between January 2013 and September 2016. Standard of care was lifelong androgen suppression with upfront docetaxel permitted from December 2015 onwards (18% of total cohort). Men allocated radiotherapy received either a daily (55 Gy in 20 fractions over 4 weeks) or weekly (36 Gy in six fractions over 6 weeks) schedule that was nominated before randomization. The median PSA was 97 ng/ml and 54% had high metastatic burden compared to 40% with low metastatic burden (6% unknown). At a median follow up of 37 months, prostate radiotherapy improved failure-free survival (HR: 0.76, 95%: CI 0.68 – 0.84; p < 0.0001) but not overall survival (HR: 0.92, 95% CI: 0.80 – 1.06; p = 0.266) in the overall cohort:
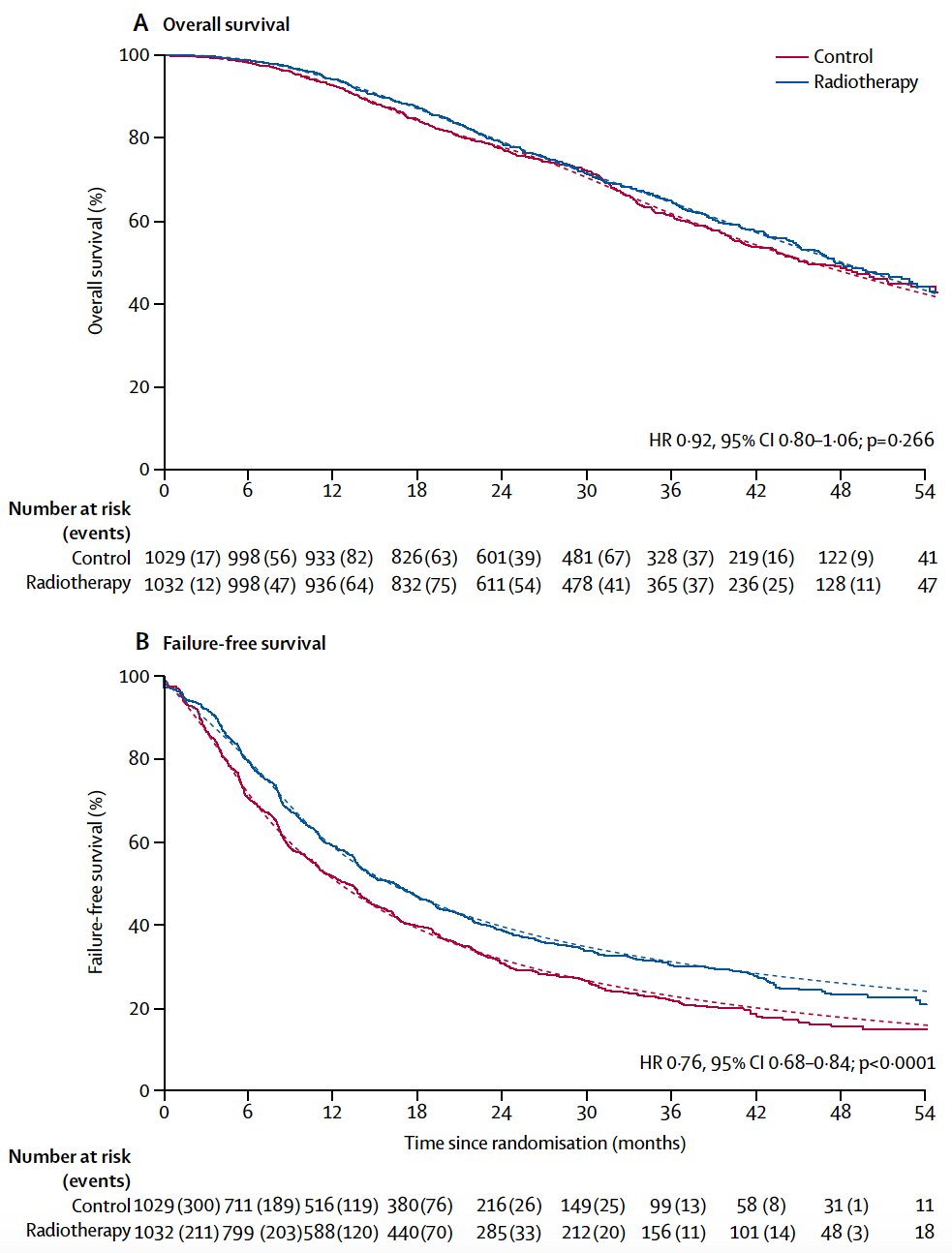
However, when stratified by metastatic burden, overall survival benefits were seen in the low volume group (HR 0.68, 95% CI 0.52 - 0.90) with restricted mean survival time improved by 3.6 months from 45.4 to 49.1. Of note, there were no significant differences between the two radiotherapy schedules, although outcomes tended to favor the daily, conventional fractionation schedule. Updated results of this trial were published in June 2022 in PLoS Medicine.3 With a median follow up of 61.3 months, prostate radiotherapy continued to demonstrate overall survival benefits in patients with low metastatic burden (HR 0.64, 95% CI 0.52 - 0.79, p < 0.001), whereas no benefit was seen in patients with a high metastatic burden (HR 1.11, 95% CI 0.96 - 1.28, p = 0.164; interaction p = 0.001).
In 2019, a systematic review and meta-analysis of the STAMPEDE Arm H and HORRAD trials was performed by the STOPCAP collaboration.4 Pooled results of 2,126 men demonstrated no overall survival improvement (HR 0.92, 95% CI 0.81-1.04) or PFS (HR 0.94, 95% CI 0.84-1.05). However, the effect of prostate radiotherapy on overall survival varied by metastatic burden (<5 versus ≥5 bone metastases: interaction HR 1.47, 95% CI 1.11-1.94, p = 0.007). There was 7% improvement in 3-year survival in men with fewer than five bone metastases.
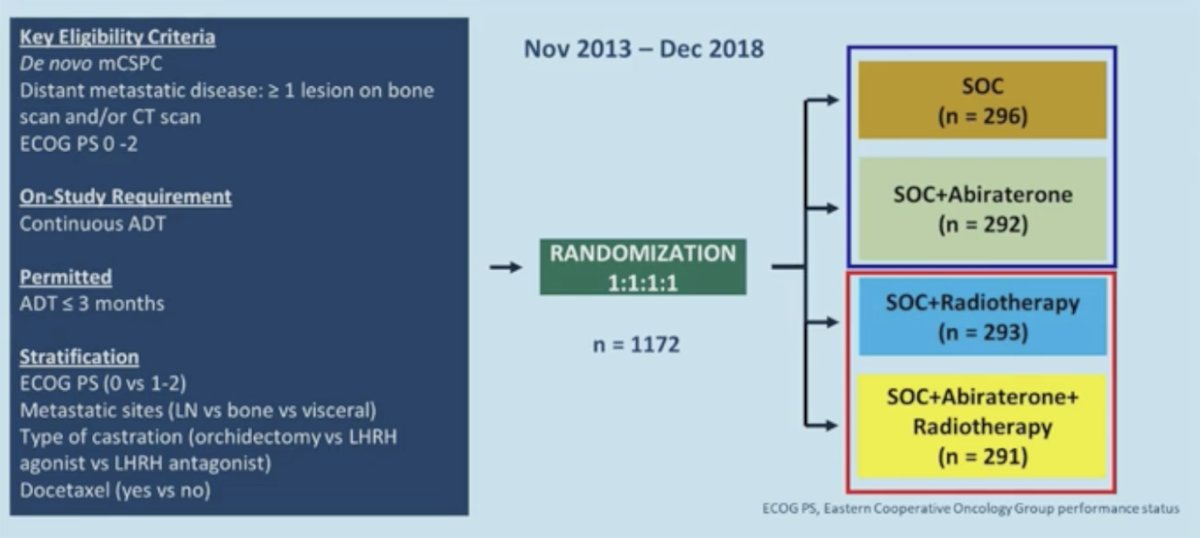
Dr. Srinivas then discussed the PEACE-1 trial,5 a phase 3 trial with a 2x2 factorial design in men with de novo metastatic castration-sensitive prostate cancer: overall survival with abiraterone acetate + prednisone. The trial design for PEACE-1 is as follows:
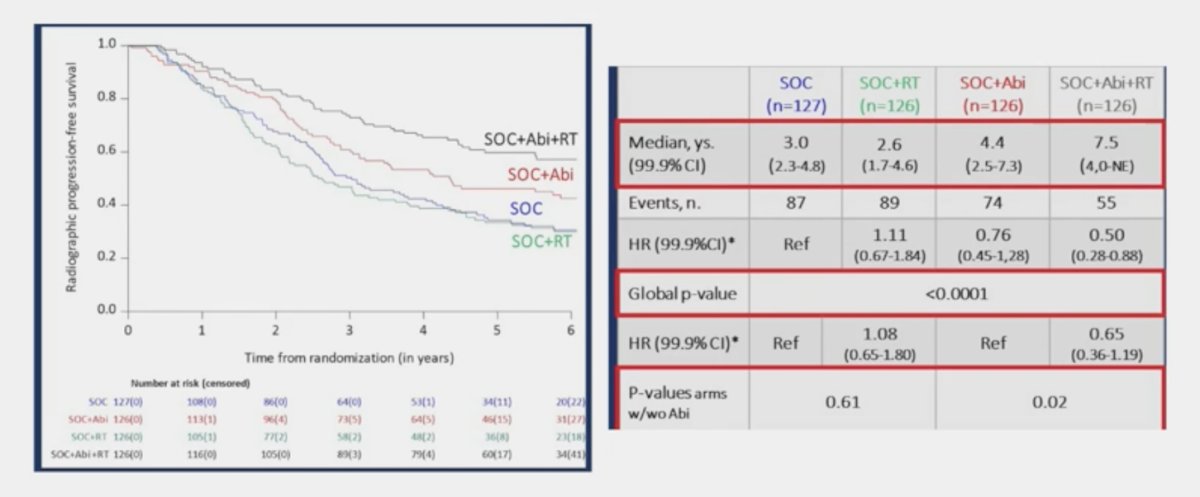
For patients with low volume disease, radiographic progression free survival was only positive in the abiraterone arm and not with ADT alone:
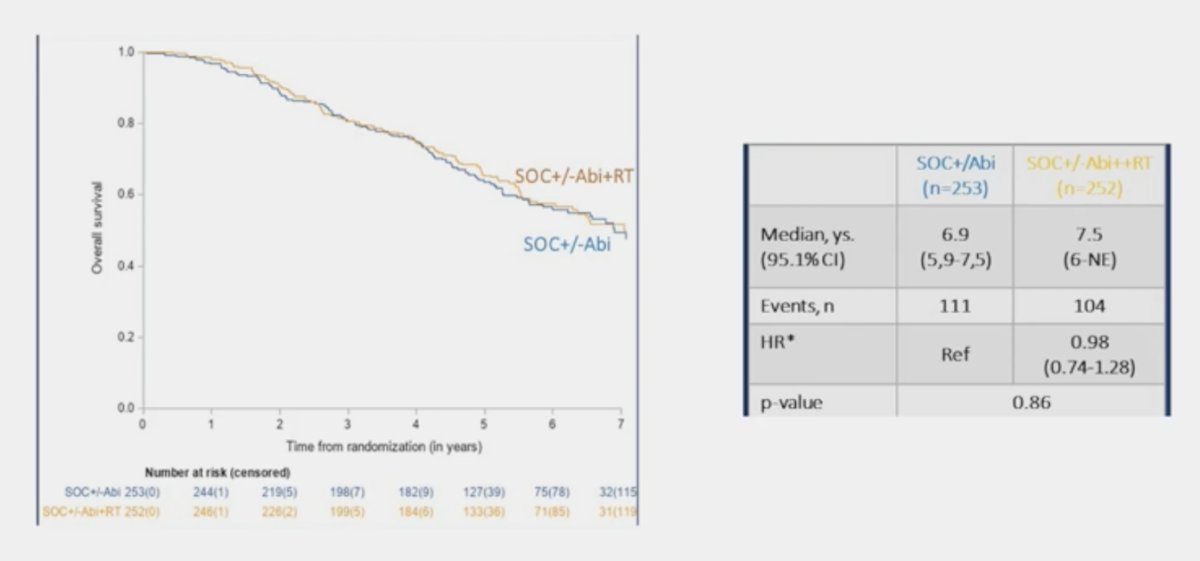
Overall survival in the low volume population assessing standard of care +/- abiraterone versus standard of care +/- abiraterone + radiotherapy showed no survival benefit with the addition of radiotherapy (HR 0.98, 95% CI 0.74-1.28):
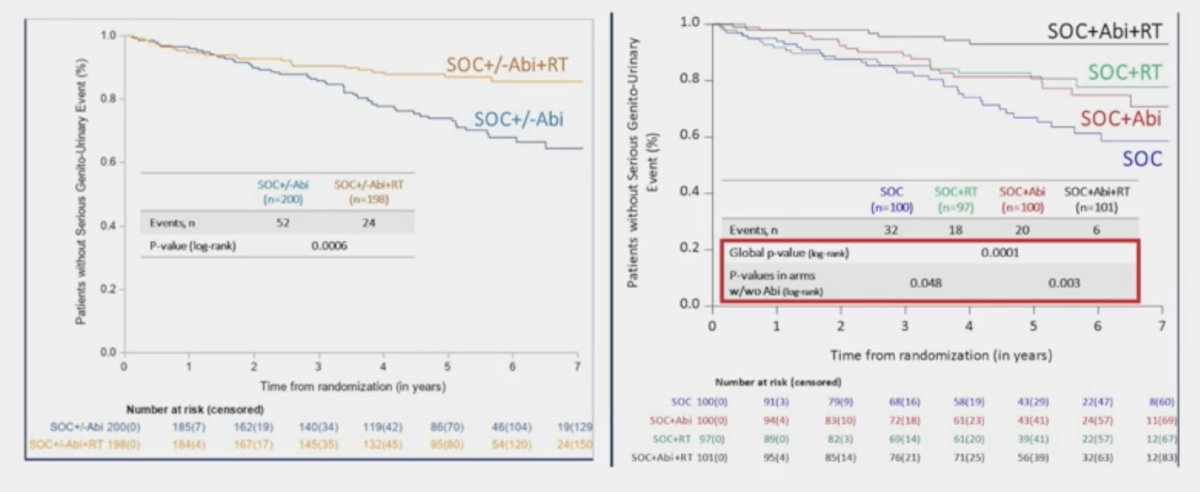
Interestingly, the addition of prostate radiotherapy to standard of care +/- abiraterone in the low-volume cohort was associated with significant improvements in the time to serious genitourinary events (p = 0.0006). This overall benefit was consistent irrespective of whether patients had prostate radiotherapy added to standard of care + abiraterone (p = 0.003) or standard of care alone (p = 0.048):
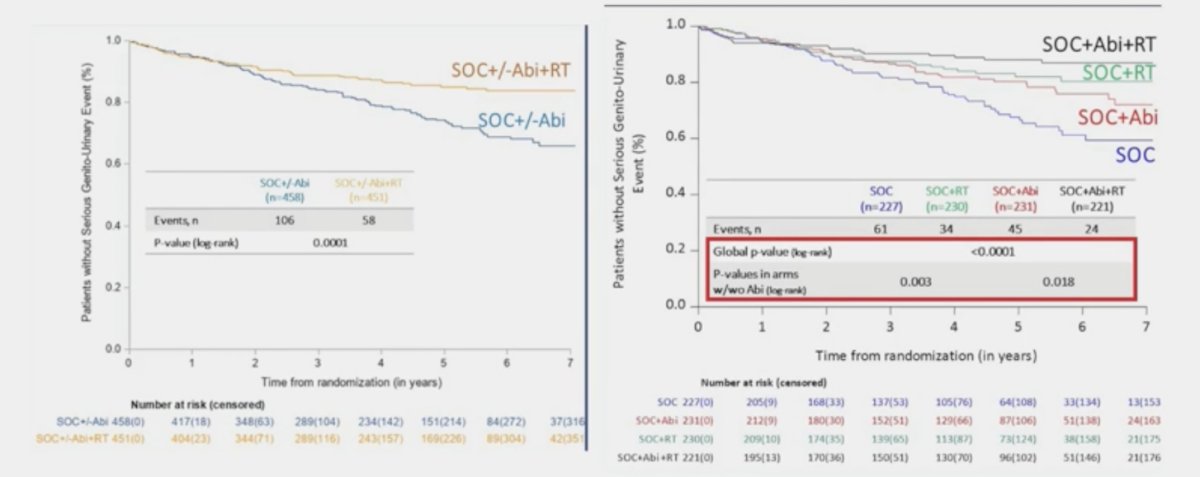
A similar benefit was observed in the overall cohort (p = 0.001):
Dr. Srinivas notes the following new learnings from the PEACE-1 trial:
- There was no difference in overall survival in low volume patients, unlike STAMPEDE
- There was improvement in rPFS seen in low volume patients, but only in the ADT + abiraterone + radiotherapy arm and not in the standard of care + radiotherapy arm, unlike STAMPEDE
- There was improvement in genitourinary related events seen in both high and low volume patients
Dr. Srinivas concluded her presentation by discussing whether the primary should be treated in synchronous high-volume mHSPC with the following messages regarding whether overall survival is needed for every intervention:
- Use of bone modifying drugs to prevent skeletal related events is widely accepted without having an impact on overall survival
- Local control to prevent bleeding, urinary retention, and catheterization is a quality of life issue
- Gleason score, volume of cancer in the prostate, and AUA symptom scores may help identify the patients with high volume disease that may benefit from local radiation
- Time to genitourinary events in PEACE-1 may help pick who lives long enough to get the genitourinary symptoms
- The current standard of care is not just ADT, so we do not know the benefit of radiotherapy in patients with treatment intensification
- Should STAMPEDE Arm G versus Arm H be looked at?
Presented by: Sandy Srinivas, MD, Stanford University, Palo Alto, CA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Advanced Prostate Cancer Consensus Conference (APCCC) Meeting, Lugano, Switzerland, Thurs, Apr 25 - Sat, Apr 27, 2024.
Related content: Radiating the Primary Tumor in Metastatic Hormone-Sensitive Prostate Cancer - Sandy Srinivas
References:
- Boeve LMS, Hulshof MCCM, Vis AN, et al. Effect on Survival of Androgen Deprivation Therapy Alone Compared to Androgen Deprivation Therapy Combined with Concurrent Radiation Therapy to the Prostate in Patients with Primary Bone Metastatic Prostate Cancer in a Prospective Randomised Clinical Trial: Data from the HORRAD Trial. Eur Urol. 2019;75(3):410-418.
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392(10162):2353-2366.
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the prostate for men with metastatic prostate cancer in the UK and Switzerland: Long-term results from the STAMPEDE randomised controlled trial. PLoS Medicine. 2022;19(6):e1003998.
- Burdett S, Boeve LM, Ingleby FC, et al. Prostate radiotherapy for metastatic hormone-sensitive prostate cancer: A STOPCAP Systematic Review and Meta-analysis. Eur Urol. 2019 Jul;76(1):115-124.
- Fizazi K, Foulon S, Carles J, Roubaud G, et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): A multicentre, open-label, randomized, phase 3 study with a 2 x 2 factorial design. Lancet. 2022 Apr 30;399(10336):1695-1707.
Related Content:


