(UroToday.com) The 2024 Advanced Prostate Cancer Consensus Conference (APCCC) meeting featured a session on the management of mHSPC, and a presentation by Dr. Gerhardt Attard discussing future changes in the setting of mHSPC. Dr. Attard split his talk into a treatment section and biomarker section, starting with treatment. Importantly, there is essentially unanimous consensus that there is no role for immune checkpoint inhibition in unselected M1 prostate cancer among men starting ADT:
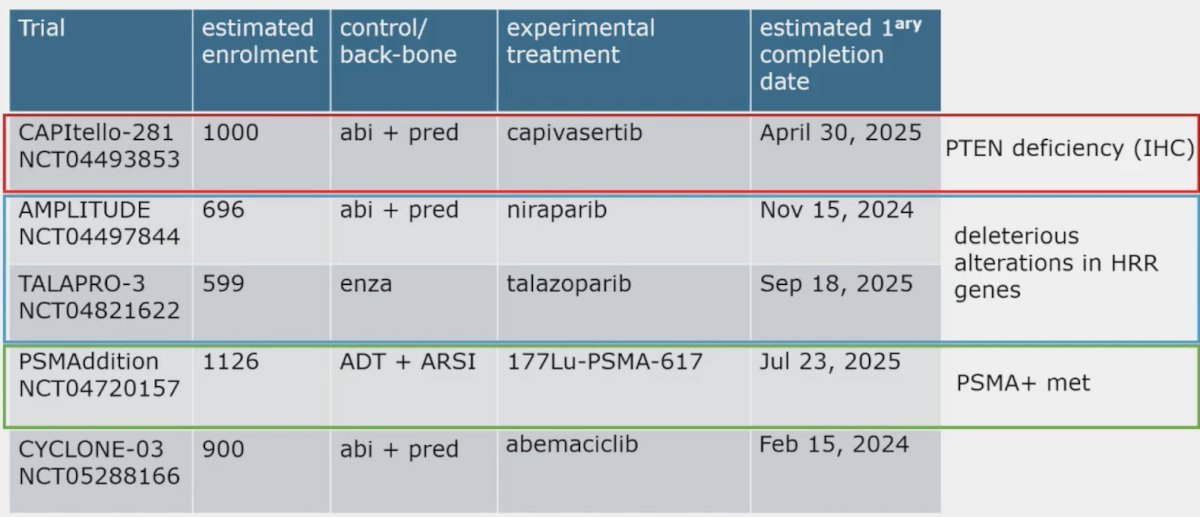
Several key phase 3 trials among men with M1 prostate cancer starting ADT are currently ongoing, including CAPItello-281, AMPLITUDE, TALAPRO-3, PSMAddition, and CYCLONE-03:
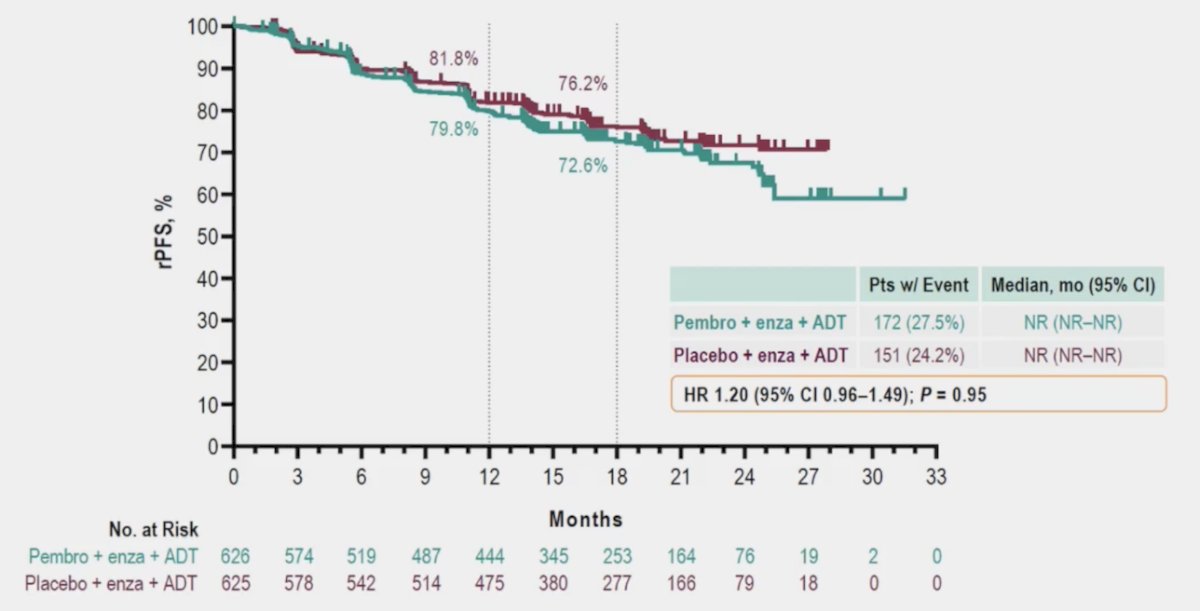
However, the future is not here yet. Dr. Attard posed the following APCCC question: In patients with synchronous mHSPC and the presence of a pathogenic BRCA alteration, does this information change your treatment recommendation for the patient?
- Yes, I recommend ADT + ARPI + docetaxel triplet systemic therapy over ADT + ARPI doublet systemic therapy regardless of disease burden
- Yes, I add platinum chemotherapy to systemic therapy regardless of disease burden
- Yes, I add a PARP inhibitor to systemic therapy regardless of disease burden
- No
The first challenge is the primary outcome measure. Phase 3 trials have primary endpoints of radiographic progression free survival, and overall survival is a secondary endpoint, thus the time frame for follow-up is 48-80 months. In recent work from Halabi et al.1 they investigated whether radiographic progression-free survival and clinical progression-free survival are valid surrogates for overall survival in men with mHSPC and could potentially be used to expedite future phase III clinical trials. Individual patient data from 6,390 patients randomly assigned from 1994 to 2012 from 13 units were pooled for a stratified analysis. The median overall survival, radiographic progression-free survival, and clinical progression-free survival were 4.3 (95% CI, 4.2 to 4.5), 2.4 (95% CI, 2.3 to 2.5), and 2.3 years (95% CI, 2.2 to 2.4), respectively. The surrogate threshold effects were 0.80 and 0.81 for radiographic progression-free survival and clinical progression-free survival endpoints, respectively:

Thus, both radiographic progression-free survival and clinical progression-free survival appear to be promising surrogate endpoints for overall survival. Dr. Attard provided the following considerations:
- mHSPC trials are experiments that should primarily test early treatment (at the start of ADT) versus late (after relapse with mCRPC)
- Caution is required when extrapolating to settings where the biologic mode of action is different
- We require surrogacy analysis using modern day trials with ADT + ARPIs (in progress with academic studies but more access to more pharma trial data is required!)
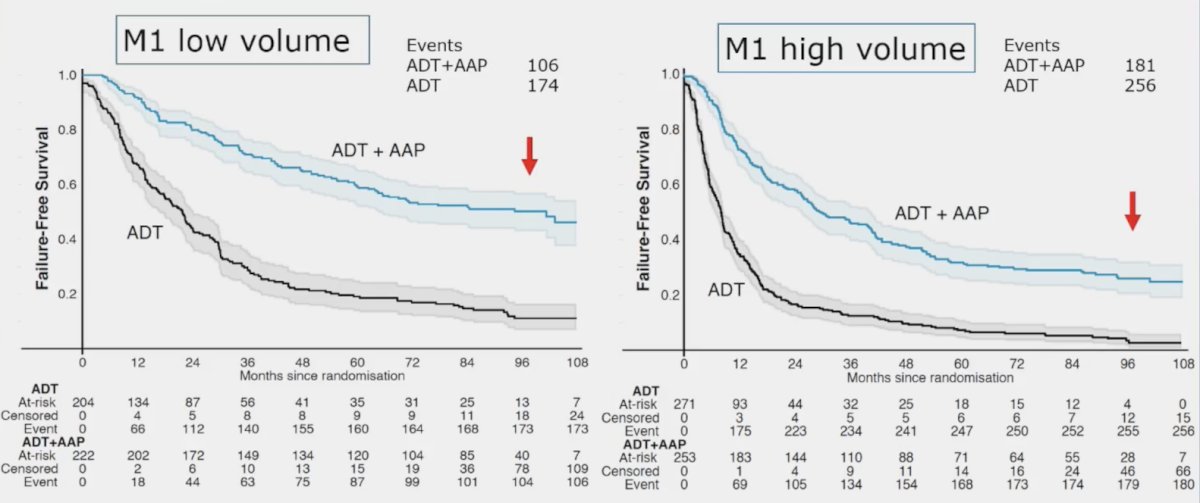
The second challenge is patient selection. In work published in 2023 from Dr. Attard’s group,2 in both M1 low volume and M1 high volume, there was a benefit for ADT + abiraterone versus ADT alone, with ~22% of patient continuing on abiraterone after > 8 years:
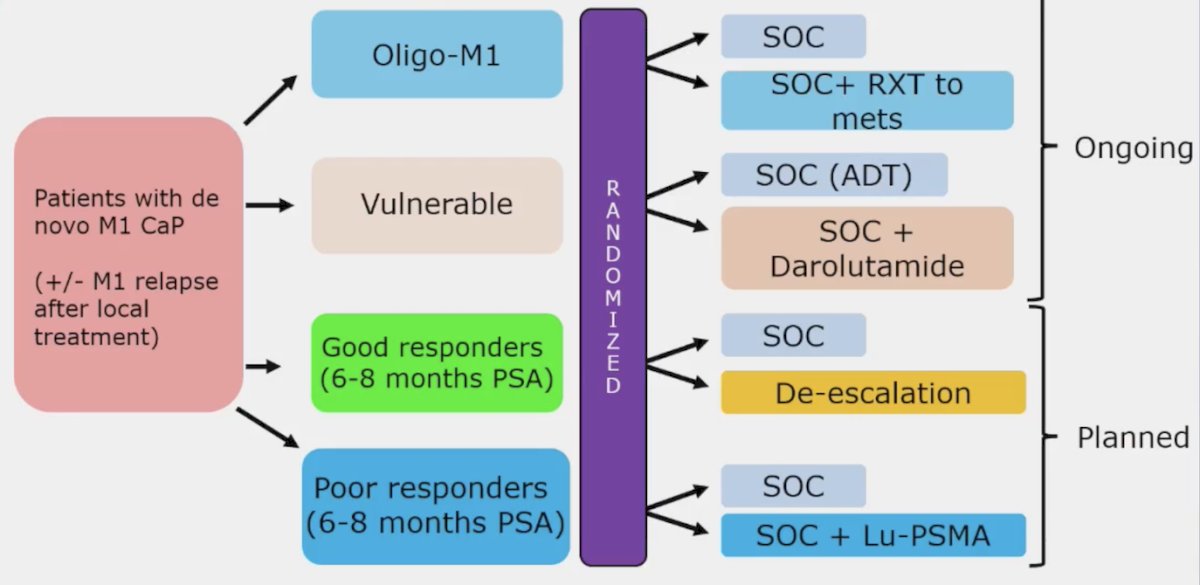
Dr. Attard notes that the PEACE-6 program is assessing multiple combinations in the mHSPC disease space, with several interesting combinations and de-escalation to provide further clarity for patient selection:
The third challenge is treatment sequencing. There are many options for treatment sequencing with two potential examples as follows:
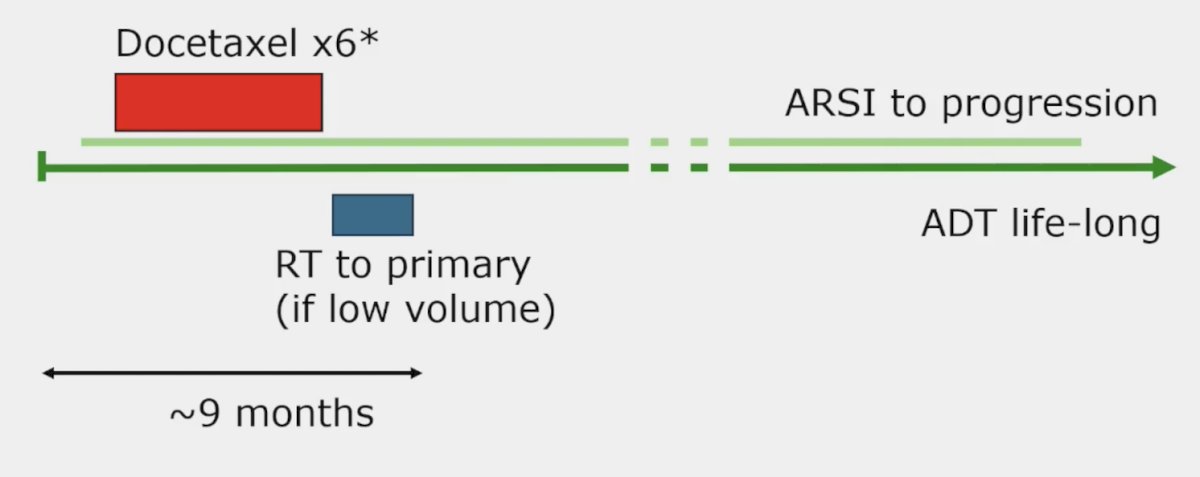
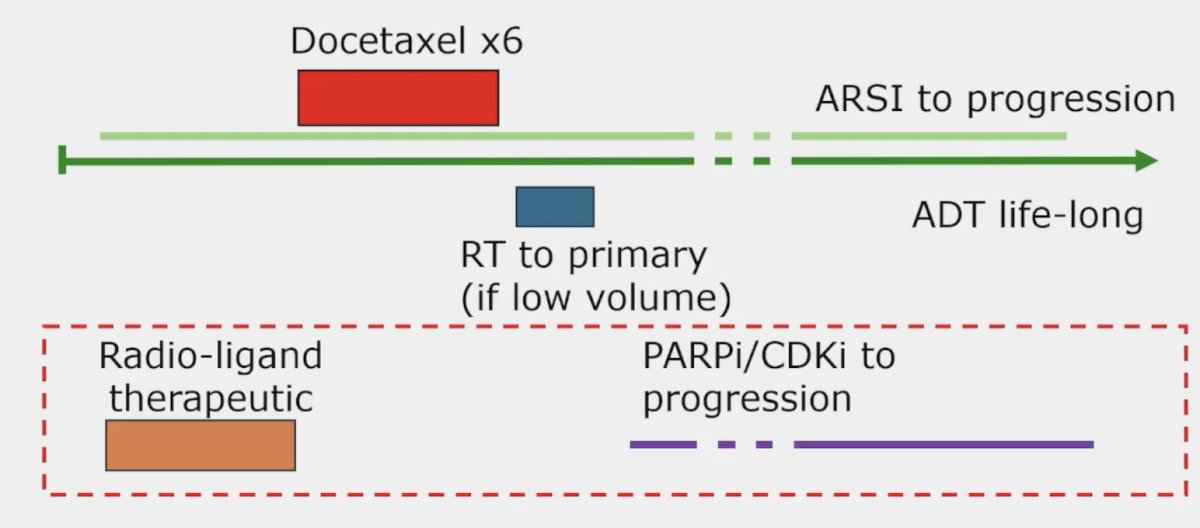
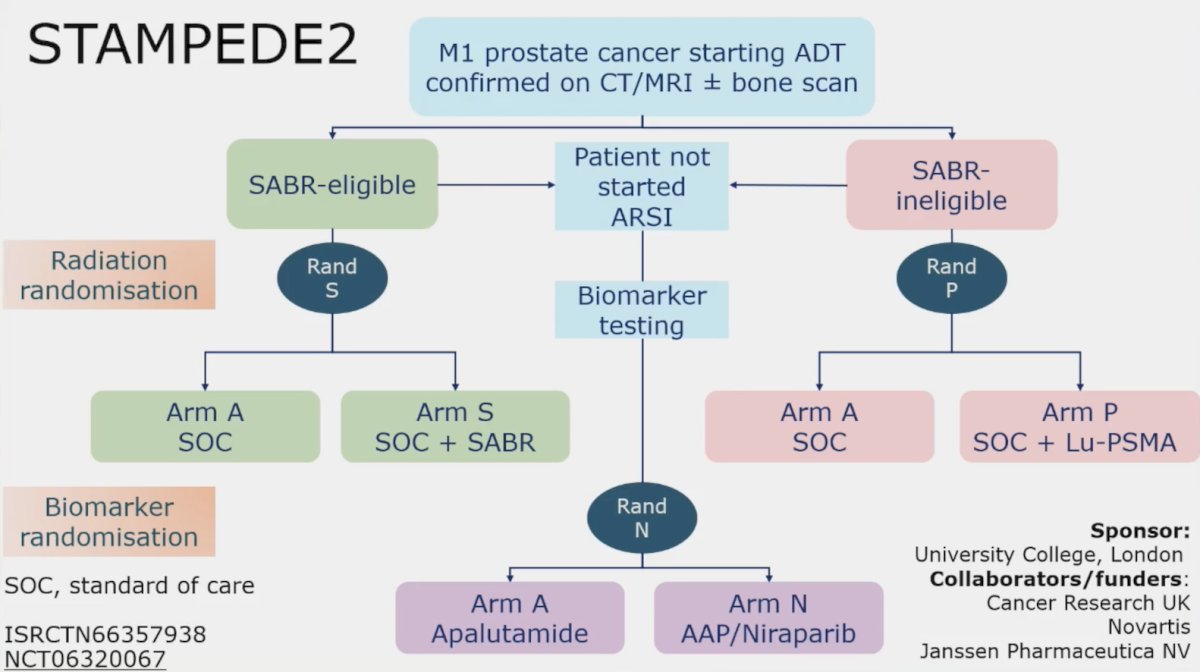
To conclude the treatment section of his talk, Dr. Attard also highlighted the STAMPEDE2 trial platform, which will incorporate PSMA PET/CT and stereotactic body radiotherapy:
Next, Dr. Attard discussed biomarkers, more specifically prognostic tests to help us assess who needs docetaxel, and predictive tests to assess if treatment will work. Biomarker discovery, validation, and implementation have several requirements:
- Large datasets with prospective follow-up
- Quality tissue requires “fit for purpose” tests
- Predictive biomarkers require directly randomized patients
- Large number of events and cross validation trials
The only way to achieve this according to Dr. Attard is for cross-consortia collaboration and utilization of the same test. In collaboration with Veracyte, there have been several studies published from the CHAARTED, STAMPEDE, and (soon to be) ENZAMET trial utilizing the Decipher genomic classifier:
At ESMO 2023, Dr. Parker presented external validation of a digital pathology-based multimodal artificial intelligence-derived model in high-risk M0/M1 prostate cancer starting ADT in the docetaxel or abiraterone phase 3 STAMPEDE trials. Of component clinical variables, p < 0.001 for prostate cancer specific mortality were PSA quartile 4 versus quartile 1-3 (HR 1.80, 95% CI 1.54-2.11), Gleason 8-10 versus <=7 (HR 1.64, 95% CI 1.36-1.98), and T4 versus T1-2 (HR 1.77, 95% CI 1.36-2.32). Additionally, the ArteraAI Prostate Test quartile 4 versus quartile 1-3 had more prostate cancer specific mortality events at 5 years: 16% (95% CI 12-19%) versus 5% (95% CI 4-7%) in M0, 58% (95% CI 53-63%) versus 39% (95% CI 36-42%) in M1:
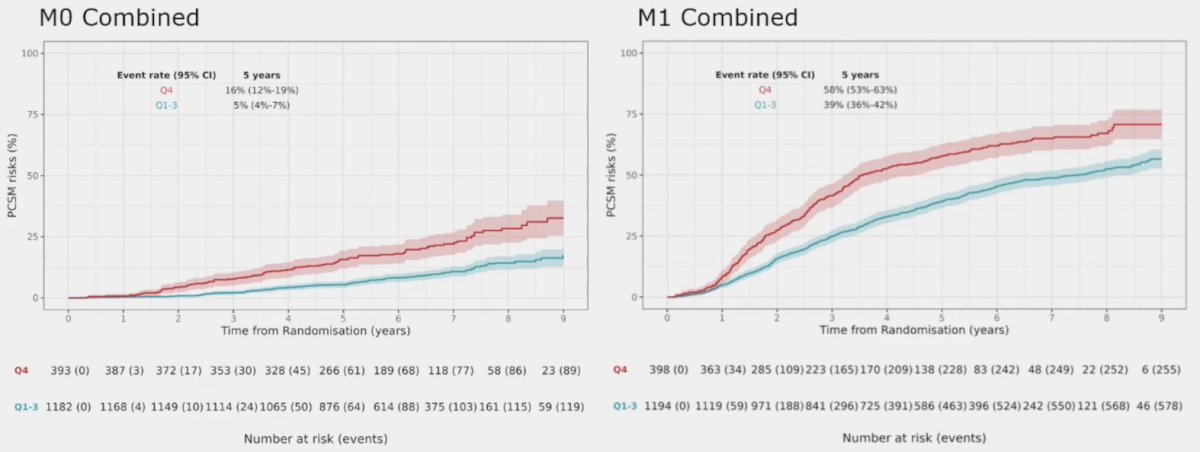
Thus, the ArteraAI Prostate Test quartile 4 identifies poor outcomes in both M0 and M1 patients. Moreover, not only does the addition of the ArteraAI Prostate Test quartile 4 identify poor prognosis patients in the high risk/metastatic setting, but it also has the following additive impact on event rate estimates for prostate cancer specific mortality at 5 years:
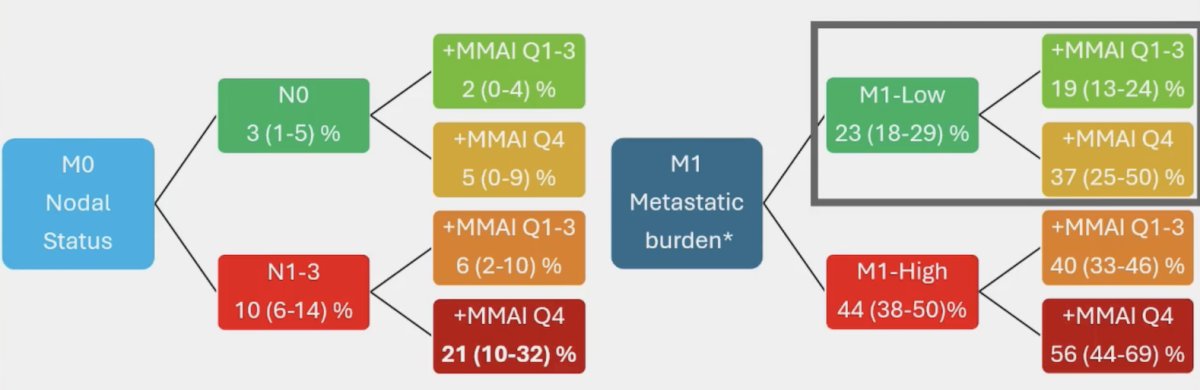
Dr. Attard concluded his presentation by discussing future changes in the setting of mHSPC with the following take-home messages:
- There are likely to be more positive phase 3 trials for radiographic progression free survival that will probably change practice, but also require follow-up for overall survival
- We need to incorporate biomarkers to guide the use of docetaxel
Presented by: Gerhardt Attard, MD, PhD, Cancer Institute and University College London Hospitals, University College London, London, UK
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Advanced Prostate Cancer Consensus Conference (APCCC) Meeting, Lugano, Switzerland, Thurs, Apr 25 - Sat, Apr 27, 2024.
References:
- Halabi S, Roy A, Rydzewska L, et al. Radiographic progression-free survival and clinical progression-free survival as potential surrogates for overall survival in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2024 Mar 20;42(9):1044-1054.
- Attard G, Murphy L, Clarke NW, et al. Abiraterone acetate plus prednisolone with or without enzalutamide for patients with metastatic prostate cancer starting androgen deprivation therapy: Final results from two randomized phase 3 trials of the STAMPEDE platform protocol. Lancet Oncol. 2023 May;24(5):443-456.


