(UroToday.com) The 2024 Advanced Prostate Cancer Consensus Conference (APCCC) meeting featured a session on the management of metastatic CRPC (mCRPC), and a presentation by Dr. Ian Davis discussing the ideal sequence after ADT alone or ADT + ARPI for treatment in the metastatic hormone sensitive prostate cancer (mHSPC) setting. Dr. Davis started his presentation by emphasizing that there is no ideal sequence after ADT alone or ADT + ARPI for mHSPC. However, there are some less than ideal sequences. The most recent updates (version 3.2024) of the NCCN prostate cancer guidelines are quite dense for mCRPC, with many options based on previous receipt of therapy:

Moreover, there is very little guidance regarding treatment duration sequencing, rather “patients can continue through all treatment options listed. Best supportive care, which can include androgen-directed therapy or steroid, is always an appropriate option.” Dr. Davis notes that there are several key principles to keep in mind:
- Only one treatment is ever given for mHSPC (unless it is changed due to toxicity)
- Every subsequent treatment is for mCRPC
- Overall diminishing benefit for later lines of therapy
- Thus, the goal is to achieve maximum benefit over the patient’s life, not just right now
- There are windows of opportunity – will an effective treatment be able to be given later?
- What is the probability of benefit given prior active treatment X has been received?
- What is “maximum benefit”?
- Delayed progression?
- Survival?
- Quality of life?
Ultimately, we need to use the most effective treatments and use them in the most effective sequence. Dr. Davis then shared the following hypothetical example based on independent versus interdependent mechanisms of action and the effect on treatment sequence:

There are many options for mCRPC, as highlighted by Dr. Davis:
- Docetaxel: TAX 3271
- Abiraterone: COU-AA-3012 and COU-AA-3023
- Enzalutamide: AFFIRM4 and PREVAIL5
- Cabazitaxel: TROPIC6 and CARD7
- Radium-223: ALSYMPCA8
- PARP inhibitor + ARPI combinations (mainly a benefit with BRCA1/2 mutation):
- PROpel9 – 22% received treatment for mHSPC, almost all docetaxel
- TALAPRO-210 - ~28% received treatment for mHSPC, almost all docetaxel
- MAGNITUDE11 - ~23% received treatment for mHSPC, mostly docetaxel
- 177Lu-PSMA:
- VISION12 and TheraP13: all pretreated for mCRPC
- ENZA-P14: limited first-line mCRPC data
As follows is the differential treatment efficacy based on clinical state, highlighting changing the biology/efficacy with treatment in the mHSPC setting by the time patients reach mCRPC:
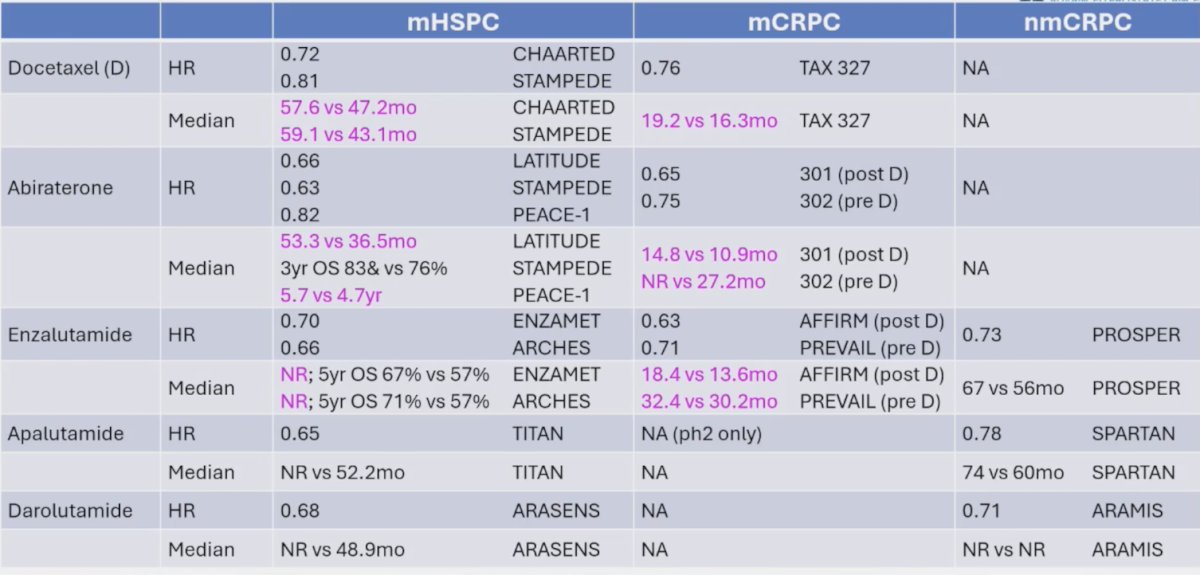
There are challenges in interpreting the literature, with variable access to therapies after mHSPC:
- mHSPC protocols do not control sequencing of post-trial therapies
- Regional and temporal variability in access to subsequent life-prolonging therapies
- Diverse patient populations:
- We cannot assume each trial is representative of the broader population
- We cannot assume trials are directly comparable
- We must consider patient specific factors
As follows are the key mHSPC trial populations and later use of life-prolonging therapies:

Additionally, there are challenges in interpreting the literature, with relevance of older mCRPC sequence data:
- Older mCRPC studies did not include patients with intensified treatment for mHSPC
- However, over half of patients today still only receive ADT alone for mHSPC
- Older reports of mCRPC sequencing:
- Docetaxel is less effective if used after ARPI, whereas cabazitaxel retains activity
- Abiraterone to enzalutamide sequence is more active than enzalutamide to abiraterone
- Real world considerations:
- Prior treatment clearly affects probability of response to later treatment
- Real world outcomes are also influenced by patient factors and accessibility to treatments
- The mCRPC median overall survival: first line is 19.4 months, second line is 14.6 months, and third line is 11.1 months
In a recent real-world study from Freedland et al.15 assessing patterns of treatment in mCRPC in the United States, they noted that baseline demographics vary, treatments were not randomized, chemotherapy was used more in younger and higher risk patients:

Generally, Europeans tended to swap mechanisms, whereas US patients tend to use another ARPI. Dr. Davis then provided thoughts on specific situations, which are not evidence based but based on his opinions:
- If a patient does not have a DDR/MMR/TMB high mutation:
- PARP inhibitors + combinations are less likely to be helpful
- There is little evidence yet for immunotherapy, but we should keep an eye on this space
- If the patient is having early/rapid progression:
- AR targeting therapy is less likely to be effective and we should consider alternative mechanisms of action
- For example, taxane chemotherapy, platinum monotherapy + combinations, radioligand therapy, and other targeted approaches
- Radioligand therapies:
- Logical and evidence-based choice for later lines of therapy
- Emerging evidence for earlier use, but caveats apply
- Combination approaches may help address tumor heterogeneity
- Always consider clinical trials
Dr. Davis concluded his presentation discussing the ideal sequence after ADT alone or ADT + ARPI for treatment in the mHSPC setting by highlighting “less than ideal” approaches in the modern area:
- Anything that means you remove a later, effective option
- This may not be obvious: missing a narrow window of opportunity to give a more toxic treatment, or use of radiation options that limit later marrow reserve capacity
- Use of a less effective treatment before a more effective one:
- Cabazitaxel before LuPSMA (TheraP)
- ARPI switch before cabazitaxel (CARD)
- Using new stuff just because it’s new or because you can:
- Why should docetaxel not be first line treatment now for mCRPC?
- Corollary: ARPI/ADT doublet for mHSPC leaves docetaxel as an option for mCRPC
- Trials designed to use ineffective / “toxic placebo” endpoints
Presented by: Ian Davis, MBBS(Hons), PhD, FRACP, FAChPM, FAHMS, GAICD, Monash University and Eastern Health, Victoria, Australia
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Advanced Prostate Cancer Consensus Conference (APCCC) Meeting, Lugano, Switzerland, Thurs, Apr 25 - Sat, Apr 27, 2024.
Related content: Sequencing Therapies in mCRPC After Progression on Combination Treatment - Ian Davis
References:
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004;351:1502-1512.
- de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995-2005.
- Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138-148.
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012;367:1187-1197.
- Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014;371(5):424-433.
- de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010;376(9747):1147-1154.
- de Wit R, de Bono J, Sternberg CN, et al. Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer. N Engl J Med 2019 Dec 26;381(26):2506-2518.
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369(3):213-223.
- Clarke N, Armstrong AJ, Thiery-Vuillemin A, et al. Abiraterone and olaparib for metastatic castration-resistant prostate cancer. NEJM Evidence 2022.EVIDoa2200043.
- Agarwal N, Azad AA, Carles J, et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): A randomized, placebo-controlled, phase 3 trial. Lancet. 2023 Jul 22;402(10398):291-303.
- Chi KN, Rathkopf D, Smith MR, et al. Niraparib and abiraterone acetate for metastatic castration-resistant prostate cancer. J Clin Oncol. 2023 Jun 20;41(18):3339-3351.
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021 Sep 16;385(12):1091-1103.
- Hofman MS, Emmett L, Sandhu S, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomized, open-label, phase 2 trial. Lancet. 2021 Feb 27;397(10276):797-804.
- Emmett L, Subramaniam S, Crumbaker M, et a. [177Lu]Lu-PSMA-617 plus enzalutamide in patients with metastatic castration-resistant prostate cancer (ENZA-p): An open-label, multicentre, randomized, phase 2 trial. Lancet Oncol. 2024 Apr 12 [Epub ahead of print].
- Freedland SJ, Davis M, Epstein AJ, et al. Real-world treatment patterns and overall survival among men with Metastatic Castration-Resistant Prostate Cancer (mCRPC) in the US Medicare population. Prostate Cancer Prostatic Dis. 2023 Oct 2 [Epub ahead of print].
Related Content:


