(UroToday.com) The 2024 Advanced Prostate Cancer Consensus Conference (APCCC) held in Lugano, Switzerland between April 25th and 27th was host to an advanced prostate cancer session with Dr. Andrew Armstrong discussing how to treat men with aggressive variant or neuroendocrine prostate cancer.
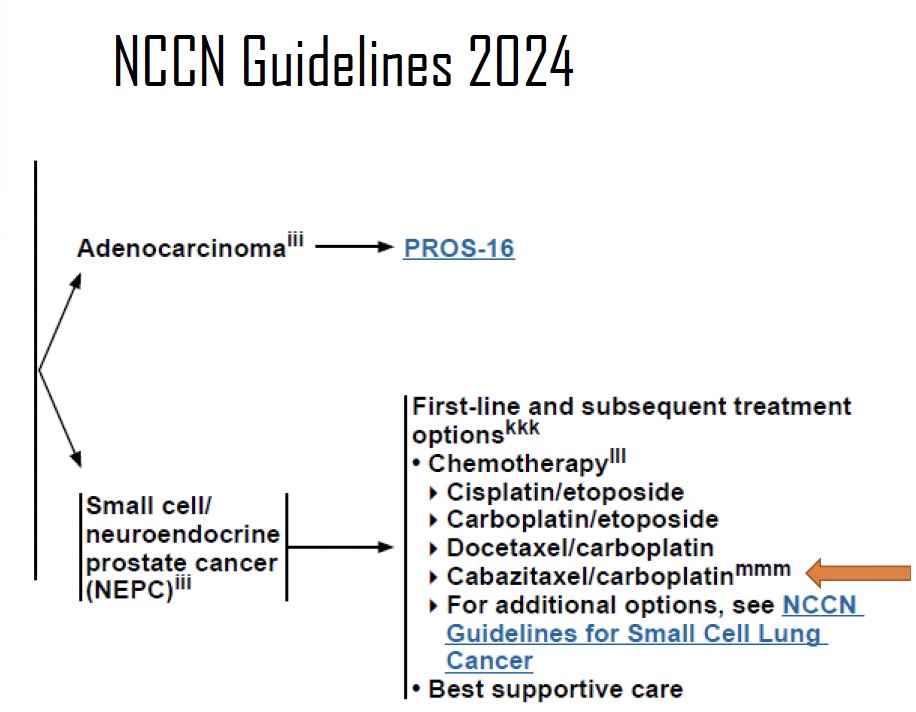
To date, there is no level 1 evidence to inform the choice of systemic therapy for these men with aggressive variant/neuroendocrine prostate cancer. Currently, the National Comprehensive Cancer Network (NCCN) guidelines recommend the following options in the 1st line and subsequent treatment settings:
- Cisplatin/etoposide
- Carboplatin/etoposide
- Docetaxel/carboplatin
- Cabazitaxel/carboplatin
- In a 2019 phase I/II trial, this combination improved median progression-free survival from 4.5 to 7.3 months (HR: 0.69, p=0.018), compared to cabazitaxel monotherapy, in pre-treated metastatic castrate-resistant prostate cancer (mCRPC) patients, with an acceptable safety profile.1
One of the challenges in managing these patients is differentiating those with neuroendocrine variants from those with other aggressive variants. Neuroendocrine variants are defined based on immunohistochemistry, circulating tumor DNA or next-generation sequencing, epigenetic profiling, and clinical criteria. This classification remains a ‘work in progress’ and patients often exist on a spectrum from adenocarcinoma to small cell carcinoma. Additionally, double negative, double positive (androgen receptor, neuroendocrine prostate cancer) tumors and patients can exist and co-exist with pure adenocarcinoma and small cell tumors and can evolve over time under different selection pressures. This is further complicated by the fact that aggressive variant prostate cancer clinical criteria do not always align with genomics or histology.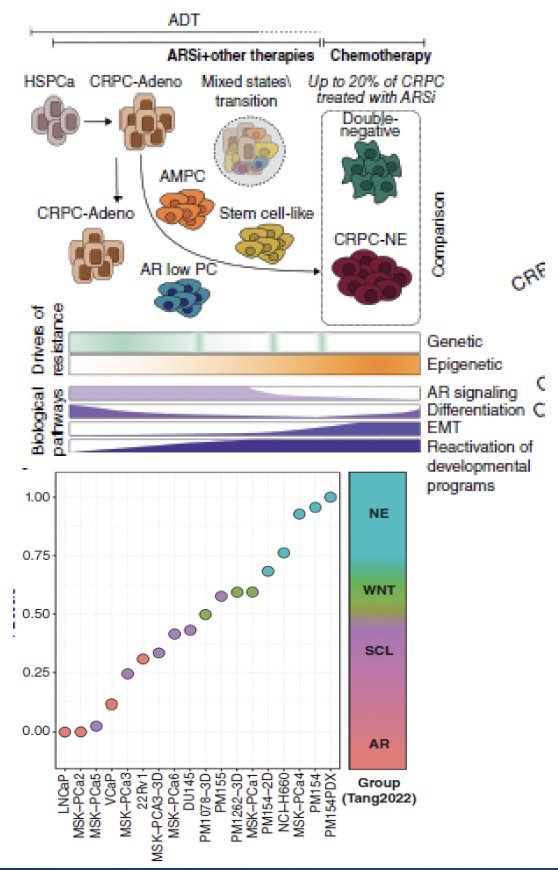
Treatment-emergent small-cell neuroendocrine prostate cancer develops in approximately 17% of patients with heavily pre-treated mCRPC. From a genomic standpoint, it appears that alterations in the DNA repair pathway are nearly mutually exclusive with treatment-emergent small-cell neuroendocrine differentiation (p=0.035). Significantly, this variant development is associated with worse overall survival outcomes (HR: 2.02, 95% CI: 1.07 – 3.82).3
Currently, level 2 evidence supports the use of platinum-based chemotherapy combinations for these patients, similar to what is recommended for patients with small-cell lung cancer. However, platinum doublets (platinum/etoposide, docetaxel/carboplatin, cabazitaxel/carboplatin) are associated with modest short-term responses (20–30%), with median progression-free and overall survivals of 4–6 and 12–18 months, respectively. Additionally, these combinations are less effective in patients with pure small-cell disease, high lactate dehydrogenase levels, and bulky disease.4
In 2019, Corn et al. published the results of the aforementioned phase I/II trial that evaluated the combination of cabazitaxel + carboplatin in patients with progressive mCRPC. In the phase II portion, 160 patients were randomly assigned (1:1) to cabazitaxel 25 mg/m2 +/- carboplatin AUC 4 mg/mL/min. At a median follow-up of 31 months, the combination improved the median progression-free survival from 4.5 to 7.3 months (HR: 0.69, p=0.018). This benefit was most notable among patients with positive clinical or molecular criteria for aggressive variant disease (small cell histology, visceral-only metastases, high lactate dehydrogenase (LDH), NEPC serum markers, bulky high-grade disease, short time to CRPC, lytic bone metastases). Overall survival benefits with this combination were only observed if there was evidence of a loss of 2/3 tumor suppressor genes (TP53, PTEN, and RB1).1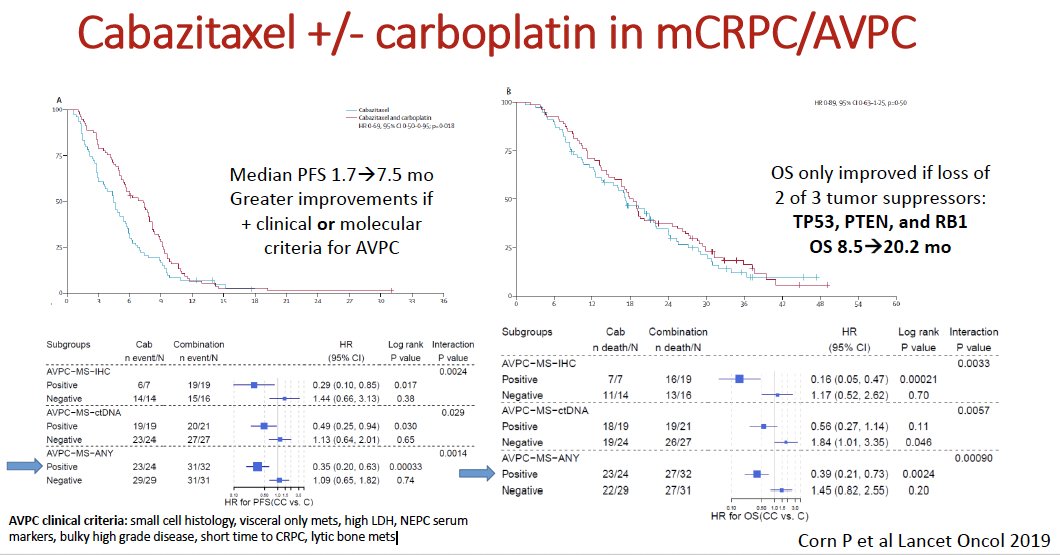
Can we further identify a cohort of neuroendocrine prostate cancer patients who have improved sensitivity to platinum-based chemotherapy? In 2021, Zhu et al. published a case series of 43 patients with neuroendocrine prostate cancer who received platinum chemotherapy and had next-generation sequencing performed. Of these 43 patients, 30% had DNA repair alterations, of whom 85% had a clinical response. This was mostly driven by those with germline or somatic BRCA2 alterations who had 100% responses. Among those without DNA repair alterations (70% of the cohort), a response was observed in only 30%. Notably, loss of TP53, RB1, or PTEN was not associated with an improved response. As illustrated in the Kaplan Meier curve below, biomarker-positive patients (i.e., presence of DNA repair alterations) had improved radiologic progression-free survival, compared to biomarker-negative patients (HR: 0.42, p=0.03; median 15 versus 7 months).
In 2018, Dr. Armstrong’s group published the results of a retrospective analysis of 73 men with mCRPC who had received platinum chemotherapy (cisplatin, carboplatin, or oxaliplatin). Patients with evidence of primary or treatment-emergent neuroendocrine prostate cancer had better clinical (63% versus 29%) and PSA50 responses (57% versus 30%), compared to those who did not. However, radiographic progression-free (5.1 versus 4.2 months) and overall survivals (8.5 versus 10 months) were similar, suggesting that histology enriches for responses but is not required for platinum sensitivity.5
What about BRCA2 and neuroendocrine prostate cancer? It appears that neuroendocrine variant may be enriched among patients with biallelic BRCA2 alterations. In a 2022 case series of 381 men with prostate cancer who underwent next-generation sequencing testing, 37 (10%) had evidence of BRCA2 alterations. Of these 37 patients, 8 (21%) had features of neuroendocrine prostate cancer. 26% of neuroendocrine prostate cancer patients had evidence of a BRCA2 loss versus a prevalence of 9% among those without this feature, suggesting enrichment for neuroendocrine/small cell transformation among BRCA2 germline or somatic carriers. This suggests the potential for PARP inhibitor therapy use among such patients in the future, with responses of 5 to 48 months to olaparib in this case series.6 Further assessment of the utility of PARP inhibitor therapy in this cohort of patients will be required.
Dr. Armstrong noted that there are similarities between intraductal/ductal histology and aggressive variant prostate cancer, namely high carcinoembryonic antigen (CEA) and LDH levels, low androgen receptor/PSA expression, and increased incidence of visceral metastases. These intraductal/ductal histologic features were enriched among patients with germline BRCA2 mutations (48% versus 12% in those without germline alterations).7 Dr. Armstrong noted that this further supports the argument for germline/somatic testing for all men with neuroendocrine and aggressive variant prostate cancer to help guide treatment choices, particularly around the benefits of platinum chemotherapy and, rarely, PD-1 blockade. While microsatellite instability (MSI) high disease is rare in neuroendocrine prostate cancer (based on anecdotal evidence and small series), he did reference a case of an exceptional responder to avelumab in the setting of MSI high mCRPC with 20% small cell carcinoma, summarized below:
What are some emerging treatment options for neuroendocrine/aggressive variant prostate cancer patients? Lurbinectedin is derived from the marine tunicate, Ecteinascidia, which inhibits oncogenic transcription and leads to double-strand DNA breaks. This drug is approved for small cell lung cancer, based on the results of a single arm, phase II trial in 105 patients that demonstrated:
- Objective response rate of 35%
- Progression-free survival: 3.5 months
- Overall survival: 9.3 months
The results of a recent retrospective, multicenter study of Lurbinectedin in 18 patients with small cell/neuroendocrine prostate cancer were presented at ASCO GU 2024 and demonstrated the following efficacy outcomes:
- Partial response: 31%
- Stable disease: 25%
- Progressive disease: 43%
- Progression-free survival: 3.4 months
- Overall survival: 6 months

There is evidence from pre-clinical models that inhibition of CXCR2 (C-X-C Motif Chemokine Receptor 2) may delay or prevent the development of neuroendocrine prostate variants in mCRPC. The combination of enzalutamide and SX-682, a CXCR2 inhibitor, is being evaluated in a multicenter phase 2 trial, SYNERGY-201, in mCRPC patients (any histology) who experience disease progression following abiraterone.
Promisingly, there are numerous ongoing clinical trials in the neuroendocrine prostate cancer disease space, as summarized below by Dr. Armstrong:
One such trial is the CHAMP trial (NCT04709276) that is evaluating the chemoimmunotherapy combination of carboplatin, cabazitaxel, nivolumab, and ipilimumab in patients with neuroendocrine/aggressive variant prostate cancer based on histology or clinical presentation. The study design is summarized below:
Additional agents being investigated in these patients include valemetostat, an EZH1/2 oral inhibitor, combination of niraparib + cetrelimab, and ADIPEG20 (PEG-arginine deiminase) maintenance.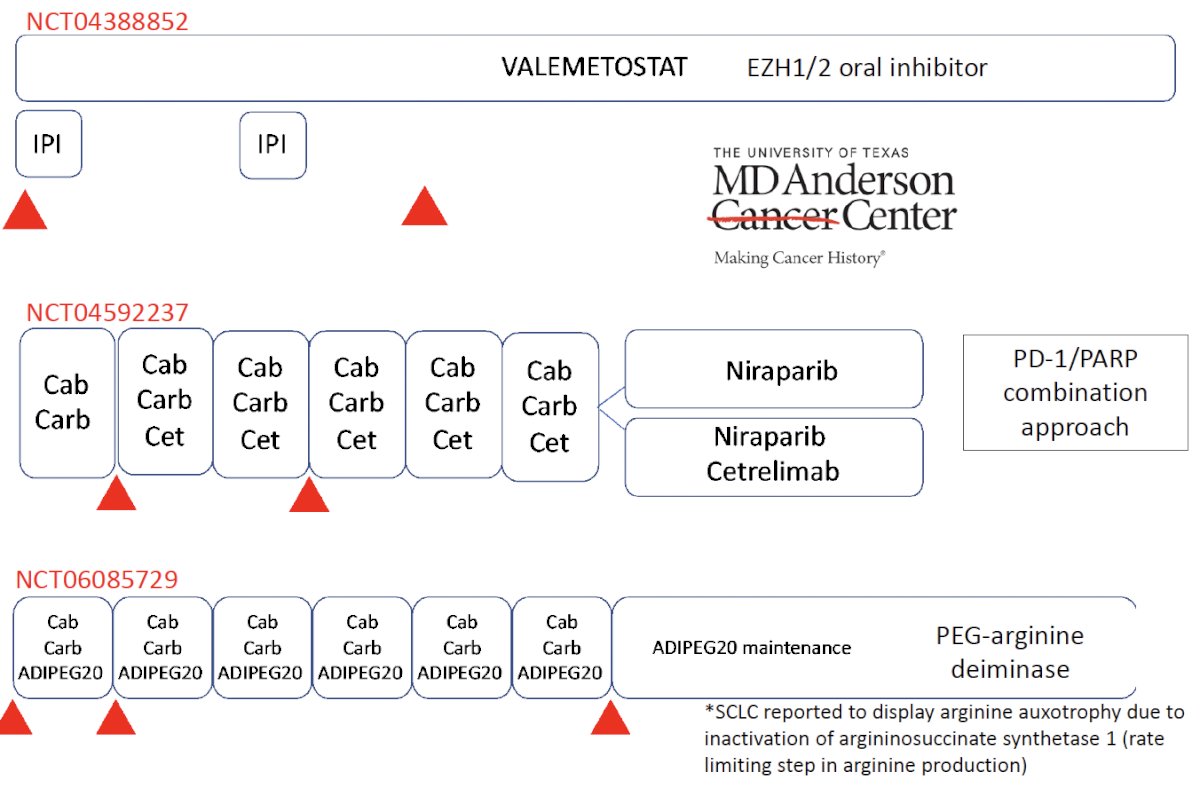
Another promising agent in this disease space is Tarlatamab (AMG-757), a potential first-in-class, investigational delta-like ligand 3 (DLL3) targeting Bispecific T-cell Engager (BiTE®) therapy for the treatment of adult patients with advanced small cell lung cancer with disease progression on or after platinum-based chemotherapy. This agent was subsequently granted FDA priority review in small cell lung cancer on December 13, 2023. Bi-specific T cell engagers targeting DLL3 have demonstrated significant in vivo activity in DLL3-high neuroendocrine prostate cancer models.8 As such, Tarlatamab (AMG 757) is currently under clinical evaluation in early-phase trials.
HPN328 is another DLL3-targeting T-cell engage with strong activity in DLL3+ cancer models.
Dr. Armstrong reiterated that one of the main challenges of treating these patients remains disease plasticity and heterogeneity. As such, combination approaches are likely needed, facilitated by the emergence of novel therapeutic targets in this disease space:
- Novel radioligand, CART, BITE, or antibody-drug conjugate targets
- GPC3, DLL3, CEACAMs, CD46 (FOR46), Bombesin (67Cu-SAR-BBN), B7-H3
- Immune evasion (CXCR2) and myeloid-derived suppressor cells (MDSC)
- SYNERGY-201 trial of SX-682 plus enzalutamide to prevent/delay CXCR2+ NEPC, and reduce MDSCs.
- Dual JAK/FGFR inhibition: Transthera’s tinengotinib
- ORR 46%, PSA50 43%, rPFS5.6 months as monotherapy
- Lurbinectadin
- Anti-MYCN therapeutics
- Epigenetic disruption and reversal of lineage plasticity
- EZH1/2, BET, LSD1, demethylation, CBP/p300 inhibition
Dr. Armstrong concluded his presentation as follows:
- Neuroendocrine/aggressive variant prostate cancer represents an aggressive phenotype of mCRPC associated with poor outcomes with conventional therapies and remains challenging to define even with tissue, blood, and clinical features.
- Repeat solid/metastatic or liquid biopsy is recommended in the mCRPC setting when neuroendocrine/aggressive variant prostate cancer is suspected clinically.
- Molecular profiling is strongly recommended in such cases given potential enrichment for HRRm (BRCA) and MSI/MMRD actionable alterations that convey PARP/platinum and PD-1 inhibitor sensitivity.
- Platinum-based chemotherapy is a consensus recommendation but results in modest response, progression-free, and overall survival improvements only in those with aggressive variant prostate cancer clinical or genomic features (2 of 3 losses in TP53/RB1/PTEN)
- Clinical trial enrollment is a consensus recommendation in these patients incorporating consensus eligibility and biomarker studies to define novel agents with potential clinical benefit for these patients.
Presented by: Andrew Armstrong, MD, ScM, FACP, Professor, Department of Medicine, Duke Health, Durham, NC
Written by: Rashid Sayyid, MD, MSc - Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 Advanced Prostate Cancer Consensus Conference, Lugano, Switzerland, April 25th - April 27th, 2024
References:
- Corn PG, Heath EI, Zurita A, et al. Cabazitaxel plus carboplatin for the treatment of men with metastatic castration-resistant prostate cancers: a randomized, open-label, phase 1-2 trial. Lancet Oncol. 2019;20(10): 1432-1443.
- Franceschini GM, Quaini O, Mizuno K, et al. Noninvasive Detection of Neuroendocrine Prostate Cancer through Targeted Cell-free DNA Methylation. Cancer Discov. 2024;14(3): 424-445.
- Aggarwal R, Huang J, Alumkal JJ, et al. Clinical and Genomic Characterization of Treatment-Emergent Small-Cell Neuroendocrine Prostate Cancer: A Multi-institutional Prospective Study. J Clin Oncol. 2018;36(24): 2592-2503.
- Aparicio AM, Harzstark AL, Corn PG, et al. Platinum-based chemotherapy for variant castrate-resistant prostate cancer. Clin Cancer Res. 2013;19(13): 3621-3630.
- Humeniuk MS, Gupta RT, Healy P, et al. Platinum sensitivity in metastatic prostate cancer: does histology matter? Prostate Cancer Prostatic Dis. 2018;21(1): 92-99.
- Symonds L, Konnick E, Vakar-Lopez F, et al. BRCA2 Alterations in Neuroendocrine/Small-Cell Carcinoma Prostate Cancer: A Case Series. JCO Precis Oncol. 2022;6:e2200091.
- Velho PI, Silberstein JL, Markowski MC, et al. Intraductal/ductal histology and lymphovascular invasion are associated with germline DNA-repair gene mutations in prostate cancer. Prostate. 2018;78(5): 401-407.
- Chou J, Egusa EA, Wang S, et al. Immunotherapeutic Targeting and PET Imaging of DLL3 in Small-Cell Neuroendocrine Prostate Cancer. Cancer Res. 2023;83(2): 301-315.


