(UroToday.com) The 2024 Advanced Prostate Cancer Consensus Conference (APCCC) held in Lugano, Switzerland between April 25th and 27th was host to an advanced prostate cancer session. Dr. Heather Cheng discussed who should undergo germline testing.
First, why is germline genetic testing important? There are numerous reasons:
- It may help the patients understand their personal risk of prostate and other cancers
- It may help their family better understand their own risks and potentially provide them with options to reduce their risk of developing cancer or diagnosing it earlier
- It may help prognosticate and identify additional treatment options for the patient’s prostate cancer
The prevalence of germline mutations varies significantly across prostate cancer disease states. As summarized in the table below, the prevalence ranges from 1.8–2.1% in patients with very low/low-risk disease to as high as 16.2% in patients with metastatic castrate-resistant prostate cancer (mCRPC). Notably, the prevalence of these germline mutations is ~ ≥10% in patients with localized, intermediate/high-risk disease, metastatic castrate-sensitive prostate cancer (mCSPC), and mCRPC. As such, Dr. Cheng argued that this high prevalence justifies germline testing in these patients. This ≥10% risk is further increased in patients with a positive family history of prostate cancer.
Dr. Cheng emphasized the importance of family history. We need to ask patients about their family history of cancers, especially prostate, breast, ovarian, pancreas, and colon. We need to inquire about both sides of the family, the age at diagnosis among those with cancers, and whether they died of their malignancy. We need to encourage patients to “know-ask-share” and enable them to take ownership.
What is the clinical actionability of such mutations for the patient? This varies by the clinical/disease setting as illustrated below: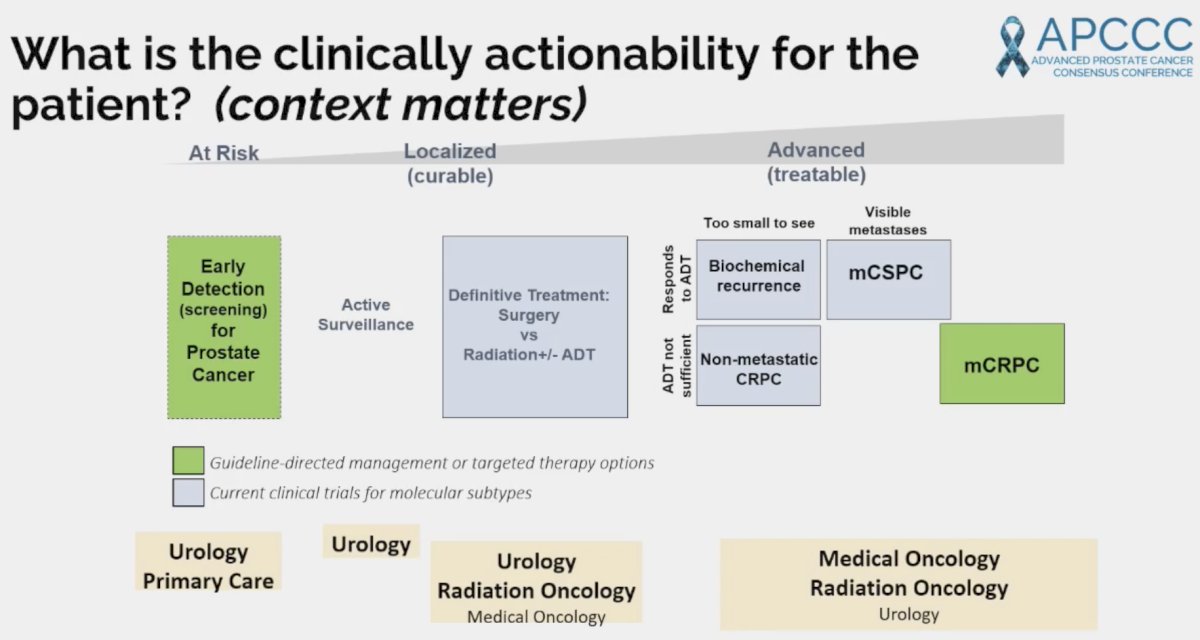
In the primary care setting, patients with germline mutations, such as BRCA1/2, ATM, and CHEK2 should be offered earlier screening, generally at 40 years of age, as per numerous guidelines.
There are currently numerous ongoing prostate cancer screening trials for patients with germline mutations:
In the advanced disease setting, the presence of germline and/or somatic mutations has important implications for treatment selection, particularly with regard to PARP inhibitors, which are now approved in the mCRPC setting both alone and/or in combination with androgen pathway inhibitors (ARPI) in patients with select homologous recombination repair mutations. These agents are being increasingly tested in earlier disease settings, including mCSPC and neoadjuvantly in localized, high-risk mutations with pathogenic mutations:
In terms of genetic counseling and the impact on family members, the 2020 ESMO Clinical Practice Guidelines note that “pathogenic mutations in cancer-risk genes identified through tumour testing should be referred for germline testing and genetic counseling”. The recently updated NCCN prostate cancer guidelines recommend that “post-[somatic] test genetic counseling and confirmatory germline testing is recommended if a mutation is identified in BRCA1, BRCA2, ATM, PALB2, CHECK2, HOXB13, MLH1, MSH2, MSH6, or PMS2. If MSI-high or dMMR is found, assess for Lynch syndrome.”
However, it is clear the genomic testing remains imperfect, which underlies the importance of a detailed clinical history. Family history still matters, and tumor testing can miss germline findings. Dr. Cheng presented a case of an 80-year-old male who was diagnosed with Grade Group 1 disease at age 61, now with mCRPC. He has a concurrent history of bladder cancer (diagnosed at 65 years of age) and papillary kidney cancer (diagnosed at 70 years of age). His family history is as follows:
- Son with pancreatic cancer (diagnosed at 40 years of age)
- Sister with glioblastoma (diagnosed at 63 years of age)
- Mother with breast cancer (diagnosed in the 60s)
- Father with jaw cancer (diagnosed in 50s) and pancreas and prostate cancers (diagnosed in 70s).
No one in the family had undergone prior germline genetic testing. In 2016, germline genetic testing of the patient did not reveal a cancer predisposition. However, in 2024, the patient was referred to the Prostate Cancer Genetics clinic and underwent in-house paired tumor/germline testing, which revealed a reduced penetrant TP53 variant. Dr. Cheng emphasized that this scenario is not uncommon in her practice, noting that TP53 is very commonly mutated in cancers, usually not germline, although observed in 0.6% of men with prostate cancer, usually as reduced penetrance variants.1 Often, there is a suspicious family history of cancer or high variant allele frequency. In a study of 100 men with advanced prostate cancer with independent germline and tumor testing, tumor-only sequencing failed to report more than 20% of pathogenic germline variants in men with advanced prostate cancer.2 As such, Dr. Cheng advised that if family history is suspicious or family implications are important to the patient, clinicians should pursue dedicated germline testing/genetic counseling.
Dr. Cheng concluded her presentation with the following take-home messages/recommendations:
- Inquire about family history of malignancies
- Offer germline testing to all men with high-risk localized, N+, and metastatic prostate cancer:
- There are numerous ongoing trials in the prostate cancer screening setting, mCSPC, and mCRPC disease states
- Those with tumor testing needing genetic counseling/germline testing have:
- Strong family history of cancer (irrespective of tumor testing)
- Tumor mutations of BRCA1/2, MLH1, MSH2, MSH6, PMS2 >> ATM, CHECK2 >>TP53
- Seek out genetics/pathologist expert (consider family history and variant allele frequency clues)
Presented by: Heather Cheng, MD, PhD, Associate Professor, Division of Hematology and Oncology, Department of Medicine, University of Washington, Seattle, WA
Written by: Rashid Sayyid, MD, MSc - Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 Advanced Prostate Cancer Consensus Conference, Lugano, Switzerland, April 25th - April 27th, 2024
References:- Maxwell KN, Cheng HH, Powers J, et al. Inherited TP53 Variants and Risk of Prostate Cancer. Eur Urol. 2022;81(3): 243-50.
- Berchuck JE, Boiarsky D, Silver R, et al. Addition of Germline Testing to Tumor-Only Sequencing Improves Detection of Pathogenic Germline Variants in Men With Advanced Prostate Cancer. JCO Precis Oncol. 2022; e2200329.


