(UroToday.com) The 2024 Advanced Prostate Cancer Consensus Conference (APCCC) held in Lugano, Switzerland between April 25th and 27th was host to an advanced prostate cancer session. Dr. Ana Aparicio discussed how non-DNA Damage Response (DDR) genomic alterations influence the management of patients with advanced prostate cancer.
There are currently numerous predictive markers for FDA-approved tumor-agnostic drugs, including:
- RET fusions: selpercatinib
- NTRK fusions: larotrectinib, entrectinib
- BRAFV600E: dabrafenib and trametinib
- HER2 overexpression: fam-trastuzumab deruxtecan
- These four mutations are however exceedingly rare in prostate cancer patients
- Microsatellite instability-high (MSI-H), tumor mutational burden high (TMB-H), DNA mismatch repair deficiency (dMMR): pembrolizumab, dostarlimab
- Relevant to patients with metastatic castrate-resistant prostate cancer (mCRPC)

Dr. Aparicio noted that the prevalence of such alterations (MSI-H and/or TMB-H) is ~3-5% and the rate of response is as high as 50%, with durable responses observed in ~25% of patients receiving pembrolizumab monotherapy. Significantly, the response rate improves as the tumor mutational burden increases.1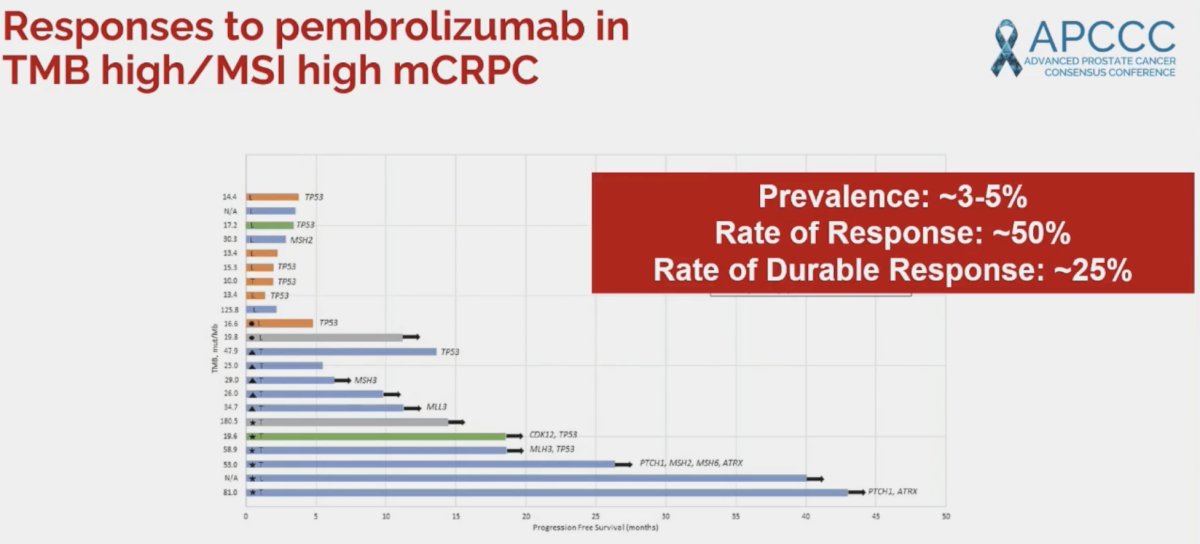
One important limitation of tumor mutational burden testing to be aware of is the variability in results across different panels, with panel size, gene content, and bioinformatics pipelines contributing to empirical variability.2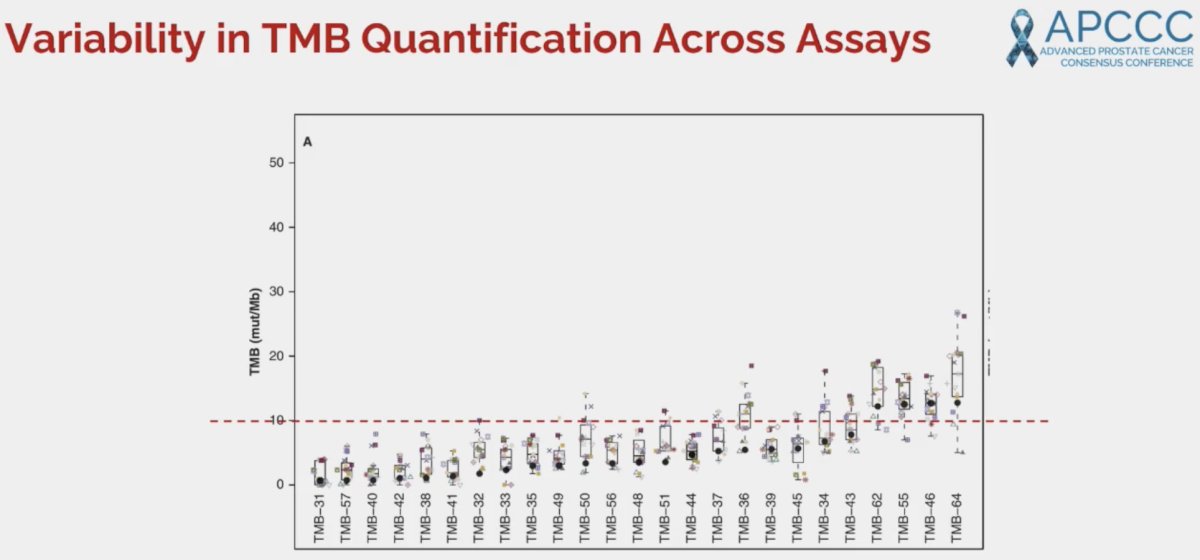
Over the past decade, numerous prognostic markers ‘specific’ to prostate cancer have been identified. These include:
- SPOP mutations
- Androgen receptor (AR) alterations
- TP53, RB1, PTEN mutations
These mutations are linked to therapy responses and outcomes. However, prospective validation of their predictive value is still needed.
Numerous studies have demonstrated that patients with SPOP mutations have superior responses to androgen axis-targeting agents. In 2022, Swami et al. demonstrated that among patients with metastatic castrate-sensitive prostate cancer (mCSPC) receiving an androgen receptor pathway inhibitor, presence of an SPOP mutation, compared with wild-type, was associated with a more favorable time to CRPC (HR: 0.20, p=0.006).3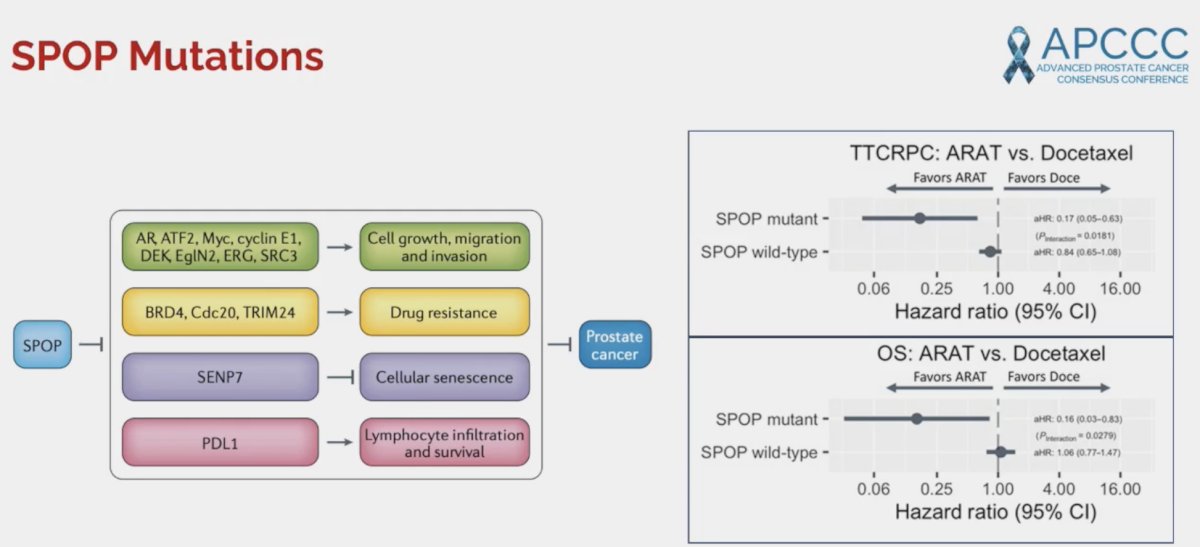
Next, Dr. Aparicio noted that AR alterations are the most frequently altered locus in mCRPC. These alterations arise following treatment exposure and are frequently subclonal in nature. The prevalence of AR alterations by disease state is summarized below:
In 2021, Annala et al. demonstrated in a cohort of 202 mCRPC patients receiving sequential AR signaling inhibitors that as the copy number alterations of AR amplifications increases, the overall survival of such patients worsens.4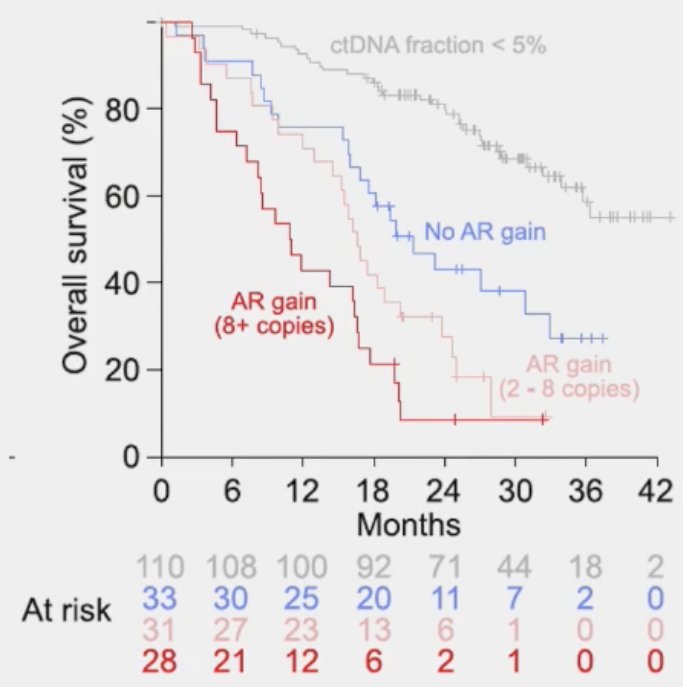
In addition to copy number alterations, the specific intragenic structural rearrangements have important prognostic implications. mCRPC patients starting abiraterone or enzalutamide with positive AR-V7 splice variants present have significantly worse progression-free and overall survival outcomes, even after adjusting for the number of circulating tumor cells and clinical prognostic factors.5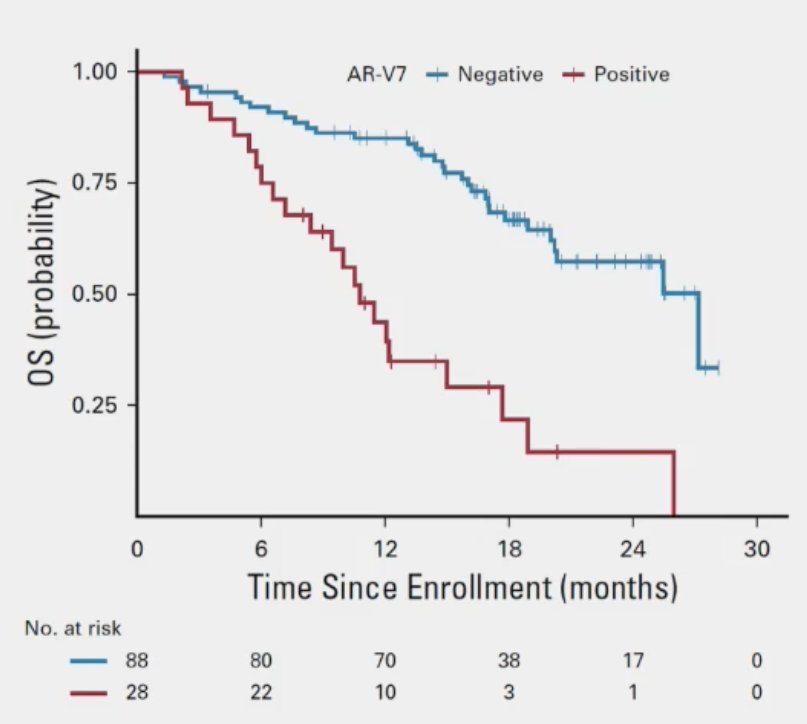
Additionally, the specific ligand-binding domain mutation has important implications for resistance to ARPIs and response to endogenous steroids.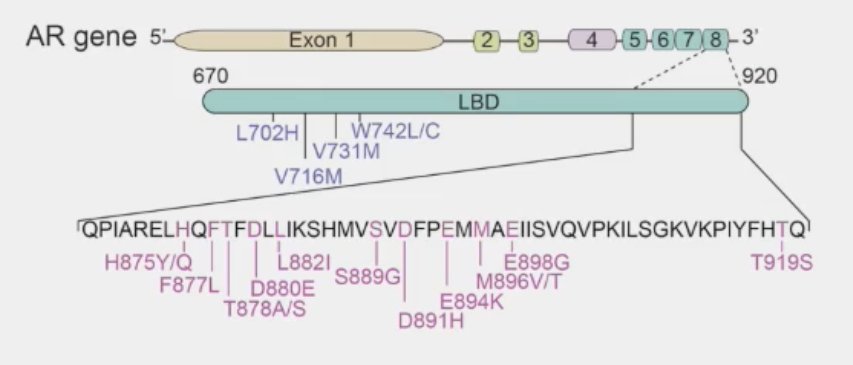
As for TP53, RB1, and PTEN mutations, Dr. Aparicio noted that these are linked to poor prognoses, both individually and when combined.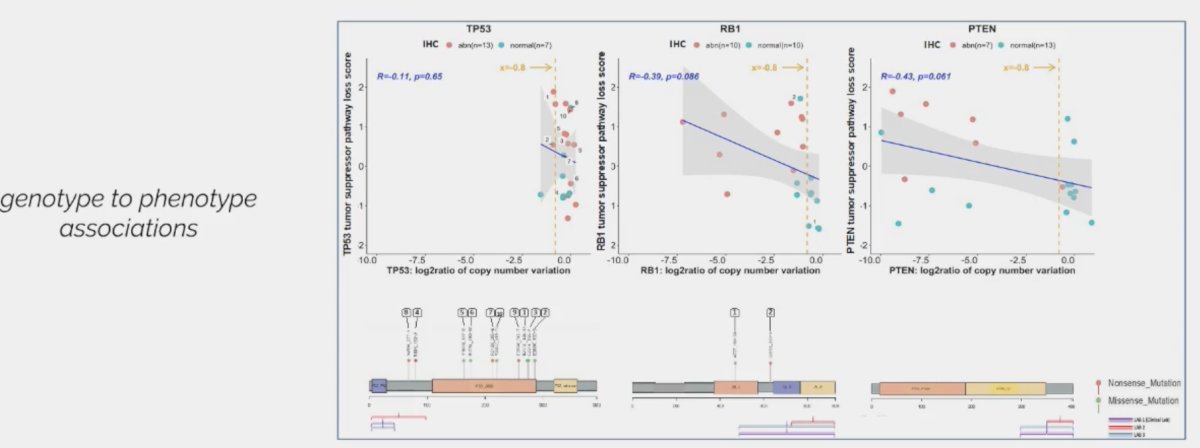
The SWOG2312 trial is evaluating cabazitaxel +/- carboplatin in mCRPC patients with aggressive variants, defined as abnormal immunohistochemistry for ≥2 of TP53, RB1, and PTEN mutations.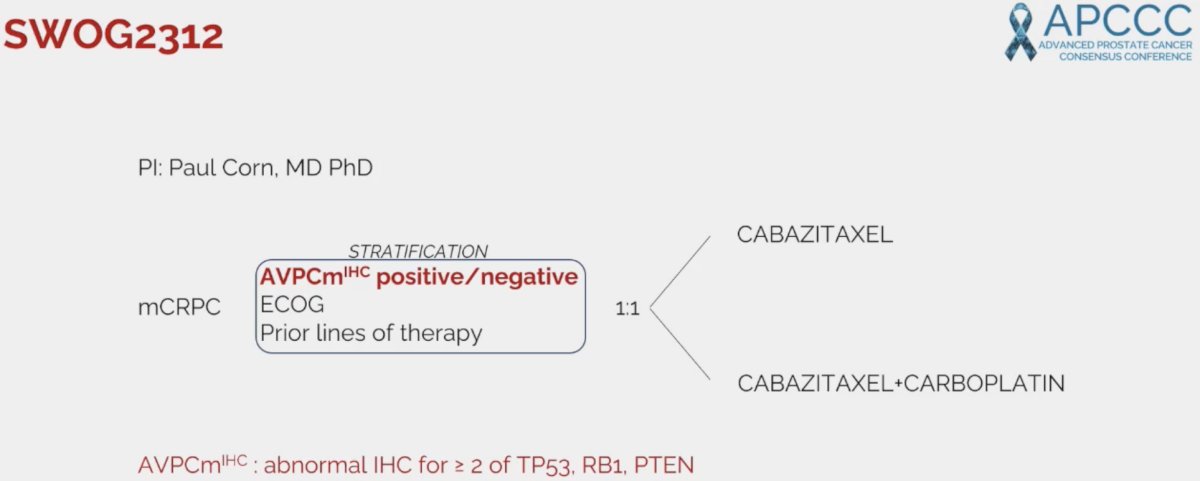
As such, a framework for therapy development in prostate cancer appears to be emerging, whereby tumors with SPOP mutations appear to be highly androgen-sensitive, those with ≥2 of TP53, RB1, and PTEN mutations being androgen resistant, and those with AR amplifications/variants falling between these two extremes.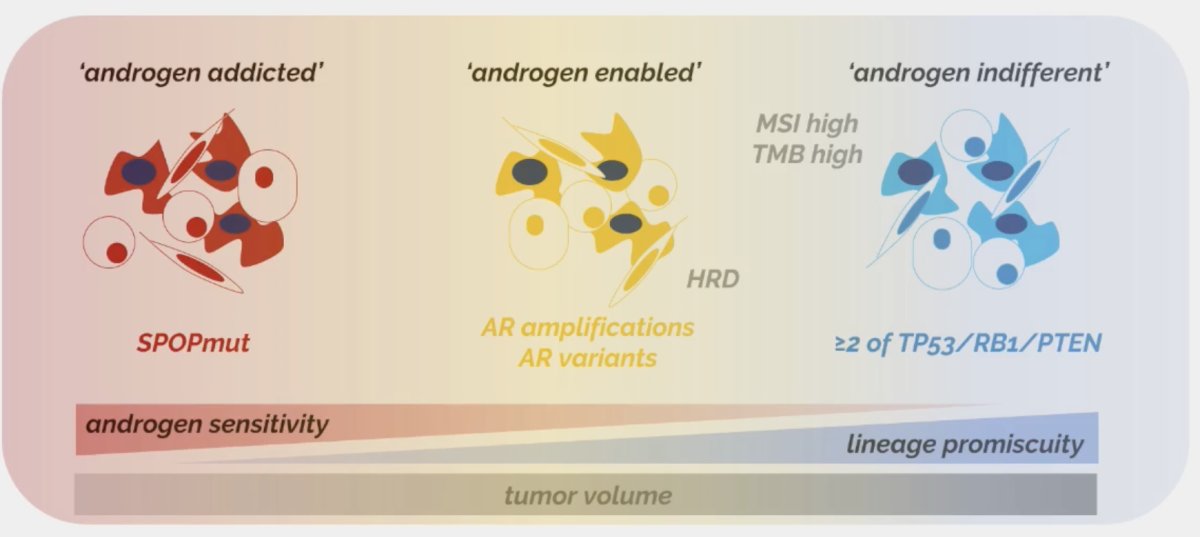
Dr. Aparicio concluded her presentation with the following take-home messages:
- Patients with microsatellite instability-high, TMB-high, mismatch repair deficient prostate cancers should be offered treatment with a PD-1 inhibitor.
- Prospective marker-selected or stratified studies are needed to validate the predictive value of other non-homologous repair deficient genomic alterations
- The clinically evident distinct biologic subsets of prostate cancer need to be accounted for in therapy development.
Presented by: Ana Aparicio, MD, Professor, Department of Genitourinary Medical Oncology, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX
Written by: Rashid Sayyid, MD, MSc - Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 Advanced Prostate Cancer Consensus Conference, Lugano, Switzerland, April 25th - April 27th, 2024
References:- Mosalem O, Tan W, Bryce AH, et al. A real-world experience of pembrolizumab monotherapy in microsatellite instability-high and/or tumor mutation burden-high prometastatic castration-resistant prostate cancer: outcome analysis. Prostate Cancer Prostatic Dis. 2024.
- Vega DM, Yee LM, McShane LM, et al. Aligning tumor mutational burden (TMB) quantification across diagnostic platforms: phase II of the Friends of Cancer Research TMB Harmonization Project. Ann Oncol. 2021;32(12): 1626-36.
- Swami U, Graf RP, Nussenzveig RH, et al. SPOP Mutations as a Predictive Biomarker for Androgen Receptor Axis-Targeted Therapy in De Novo Metastatic Castration-Sensitive Prostate Cancer. Clin Cancer Res. 2022;28(22): 4917-25.
- Annala M, Taavitsainen S, Khalaf DJ, et al. Evolution of Castration-Resistant Prostate Cancer in ctDNA during Sequential Androgen Receptor Pathway Inhibition. Clin Cancer Res. 2021;27(16): 4610-23.
- Armstrong AJ, Halabi S, Luo J, et al. Prospective Multicenter Validation of Androgen Receptor Splice Variant 7 and Hormone Therapy Resistance in High-Risk Castration-Resistant Prostate Cancer: The PROPHECY Study. J Clin Oncol. 2019;37(13): 1120-9.
Related Content:


