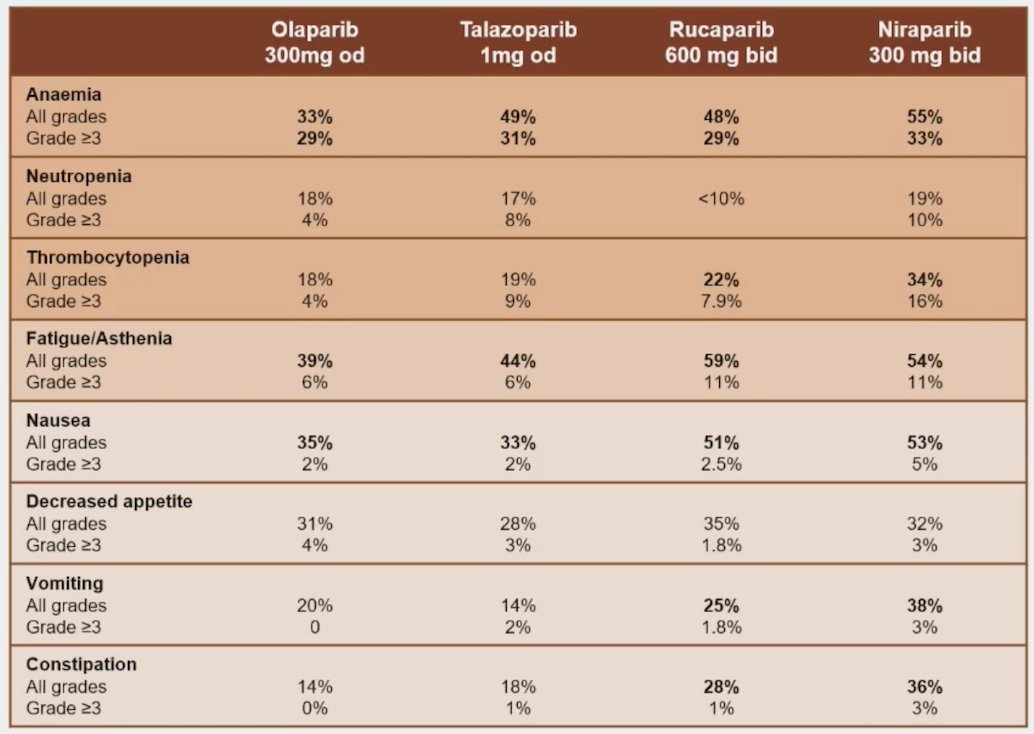
(UroToday.com) The 2024 Advanced Prostate Cancer Consensus Conference (APCCC) meeting featured a session on the management of metastatic CRPC (mCRPC), and a presentation by Dr. Elena Castro discussing how to manage side effects of PARP inhibitors. Dr. Castro notes that in the PARP inhibitor monotherapy trials, there were frequent adverse events:
Comparatively, there were also frequent adverse events in the ARPI + PARP inhibitor combination trials, although there were no differences in toxicity by HRR status:
Of note, frequent side effects tend to occur early in the treatment course. When looking at the three PARP inhibitor + ARPI combination trials (MAGNITUDE,1 PROpel,2 and TALAPRO-2),3 Dr. Castro highlighted that there are several adverse events of special interest. Anemia is arguably the most common adverse event grade >= 3, ranging from 16% in the PROpel trial to 46% in the TALAPRO-2 trial. Hypertension was also notable in the MAGNITUDE trial at 15% grade >= 3. Moreover, it appeared that venous thromboembolism was frequent, including 4.7% of patients in MAGNITUDE with a pulmonary embolism, 7% in PROpel, and 3% in TALAPRO-2. A side-by-side comparison of the three trials comparing adverse events is as follows:
Knowing how to manage hematological side effects from PARP inhibitors is important and is based on the grade of adverse event. Patients with grade 1 anemia (hemoglobin >= 10 g/dL) are monitored, whereas those with grade 2 anemia (hemoglobin <10 - >= 8 g/dL) should have a dose interruption, transfusions till the hemoglobin reaches 9 g/dL, and frequent monitoring. No dose reduction is required when the patient is started back on therapy. Grade 3-4 anemia (hemoglobin < 8 g/dL) is managed similarly to grade 2 anemia, but the dose is reduced when restarted. The following treatment algorithm also highlights the management for subsequent occurrences: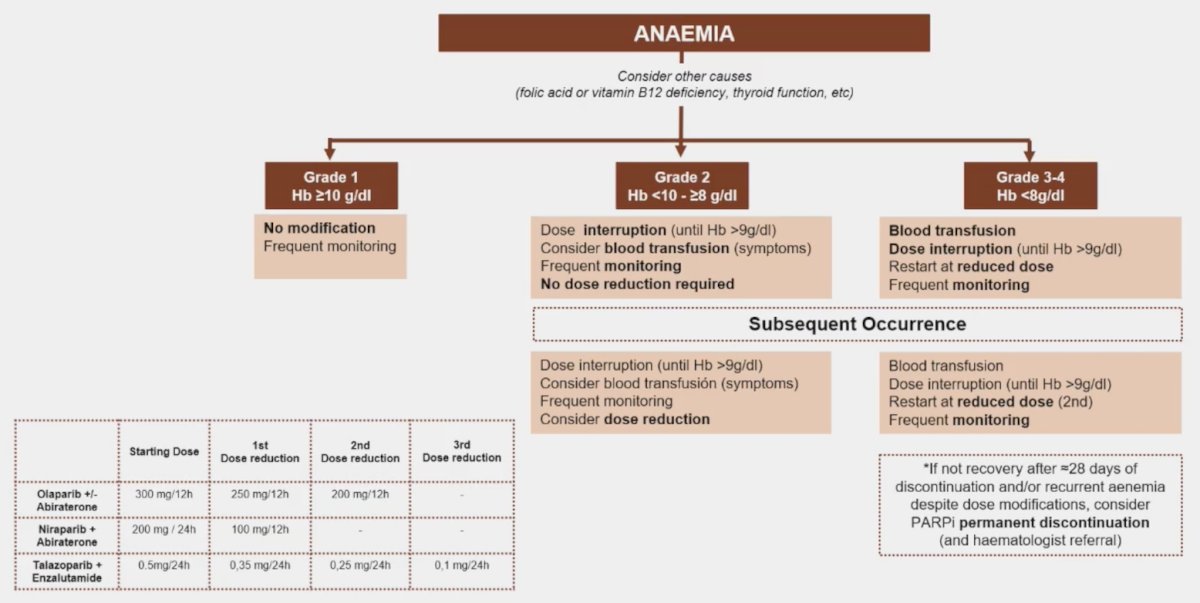
The management of neutropenia, leukopenia, and thrombocytopenia is as follows:
Dr. Castro also mentioned management options for non-hematologic side effects:
- Nausea/vomiting: prokinetics and 5-HT3 receptor antagonists, anti-emetics, light meals with an anti-emetic before treatment, and avoidance of a CYP3A4 inhibitor with olaparib
- Diarrhea/constipation: loperamide or laxatives
- Fatigue: non-pharmacologic treatments (physical activity), psychostimulants for more symptomatic patients
- Hypertension: antihypertensives
- Renal toxicity: an increase in creatinine may not reflect a true decline in GFR (ie. rucaparib can inhibit the renal transporters MATE1 and MATE2)
Generally, the non-hematological adverse events are handled as follows, also based on the grade of severity:
Myelodysplastic syndromes are clonal hematopoietic neoplasms characterized by the combination of persistent unexplained cytopenias and morphologic dysplasias, and a propensity to progress to bone marrow failure or acute myeloid leukemia. Acute myeloid leukemia is a clonal expansion of immature “blast cells” in the peripheral blood and bone marrow resulting in ineffective erythropoiesis and bone marrow failure (cytopenias). Risk factors for developing these entities include age > 60 years, genetic predisposition, chemotherapy, and environmental exposure. With regards to PARP inhibitor treatment and development of these conditions, Dr. Castro recommends the following:
Dr. Castro then discussed a recent meta-analysis assessing myelodysplastic syndrome and acute myeloid leukemia in patients treated with PARP inhibitors.4 Among 18 randomized clinical trials (n = 7,307 patients), PARP inhibitors significantly increased the risk of myelodysplastic syndrome and acute myeloid leukemia compared with placebo treatment (OR 2.63, 95% CI 1.13-6.14, p = 0.026). The incidence of myelodysplastic syndrome and acute myeloid leukemia across PARP inhibitor groups was 0.73% (95% CI 0.50-1.07; 21 events out of 4,533 patients) and across placebo groups was 0.47% (0.26-0.85; three events out of 2,774 patients).
In this same publication,4 a review of the WHO VigiBase database (cut off May 3, 2020) was undertaken, with a reported 178 cases of myelodysplastic syndrome (n = 99) and acute myeloid leukemia (n = 79) related to PARP inhibitors,4 including 7 for patients with breast cancer, 119 for ovarian cancer, 3 for pancreatic cancer, 10 for prostate cancer, and 1 for vulvar cancer. The median age at diagnosis was 64 years (range: 38-81), the median PARP inhibitor duration was 9.8 months (range: 3.6 – 17.4), and the latency period from first PARP inhibitor exposure to myelodysplastic syndrome was 17.8 months, and was 20.6 months for acute myeloid leukemia. Dr. Castro notes that this is a shorter latency period than with conventional chemotherapy, which is typically 3-5 years.
Dr. Castro concluded her presentation discussing how to manage side effects of PARP inhibitors with the following take-home messages:
- In BRCA/HRR patients, PARP inhibitor benefits largely outweigh the risks associated with potential adverse events
- Most toxicities are acute and can be safely managed, however, regular monitoring is needed (particularly in the first 3-4 months)
- Myelodysplastic syndrome and acute myeloid leukemia are delayed side effects
- They are infrequent, although the true incidence may be underestimated
- Should be suspected in the event of persistent/recurrent cytopenias
- Should be considered for future trials/treatment strategies
Presented by: Elena Castro, MD, PhD, Medical Oncologist, Hospital Universitario 12 de Octubre, Madrid, Spain
Written by: Zachary Klaassen, MD, MSc - Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Advanced Prostate Cancer Consensus Conference (APCCC) Meeting, Lugano, Switzerland, Thurs, Apr 25 - Sat, Apr 27, 2024.
References:
- Chi KN, Rathkopf D, Smith MR, et al. Niraparib and abiraterone acetate for metastatic castration-resistant prostate cancer. J Clin Oncol. 2023 Jun 20;41(18):3339-3351.
- Saad F, Clarke NW, Oya M, et al. Olaparib plus abiraterone versus placebo plus abiraterone in metastatic castration-resistant prostate cancer (PROpel): final prespecified overall survival results of a randomized, double-blind, phase 3 trial. Lancet Oncol. 2023 Oct;24(10):1094-1108.
- Agarwal N, Azad AA, Carles J, et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): A randomized, placebo-controlled, phase 3 trial. Lancet. 2023 Jul 22;402(10398):291-303.
- Morice PM, Leary A, Dolladille, et al. Myelodysplastic syndrome and acute myeloid leukemia in patients treated with PARP inhibitors: A safety meta-analysis of randomized controlled trials and a retrospective study of the WHO pharmacovigilance database. Lancet Haematol. 2021 Feb;8(2):e122-e134.
Related Content:


