(UroToday.com) The 2024 Advanced Prostate Cancer Consensus Conference (APCCC) meeting featured a session on the management of Metastatic Castration Resistant Prostate Cancer (mCRPC), and a presentation by Dr. Joaquin Mateo discussing the best use of PARP inhibitors in mCRPC. The challenge of precision medicine is that PARP inhibitors are (mostly) in tumor cells deficient for HRR and we do not currently have tests to measure HRR function. As such, we use “proxy” biomarkers, which are different from targeting the oncogene:

Dr. Mateo emphasized that BRCA-deficient metastatic prostate cancer is a distinct disease subset with a poor prognosis:
But, BRCA-deficient metastatic prostate cancer is a disease that has high sensitivity to PARP inhibition, as previously shown in the TOPARP-B trial of olaparib monotherapy:1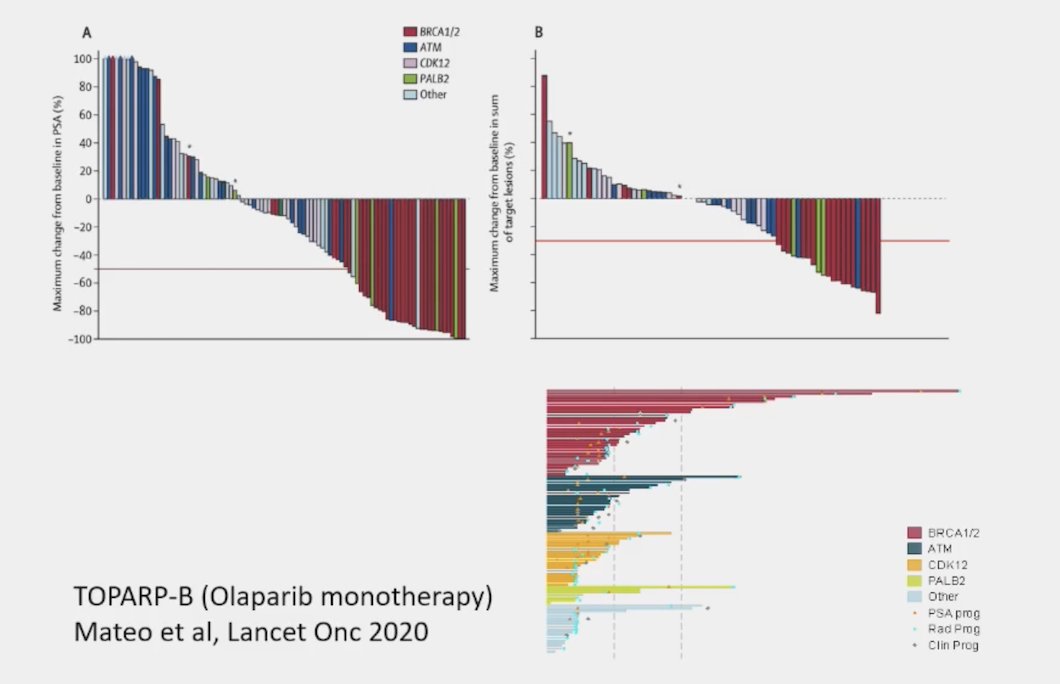
These findings in TOPARP-B were subsequently confirmed in the PROFOUND trial2 assessing olaparib versus ARPI in mCRPC BRCA 1/2 patients, and the TRITON 3 trial3 assessing rucaparib versus physician’s choice of ARPI or taxane in mCRPC BRCA 1/2 patients: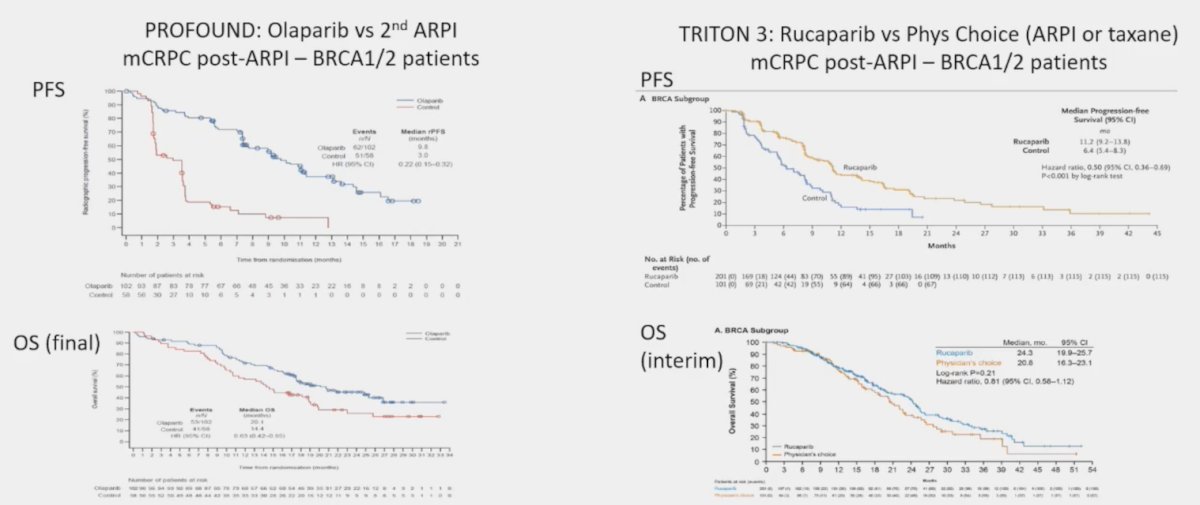
There remain several key questions. The first key question is whether there is different efficacy for BRCA1 versus BRCA2 mutations. The prevalence of BRCA1 in mCRPC is 1-2%, whereas for BRCA2 is 8-10%. Generally, based on data from TRITON24 and PROFOUND, responses are more robust in patients with BRCA2 mutations: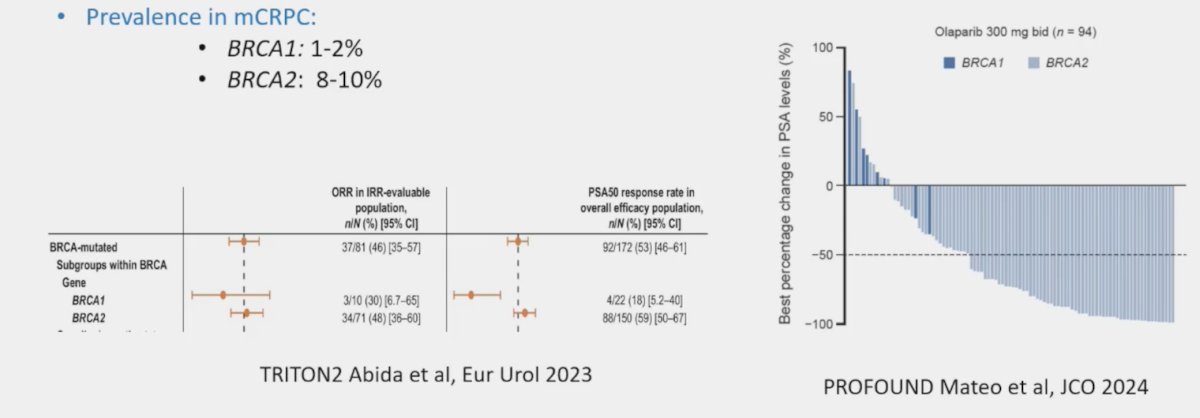
Importantly, Dr. Mateo notes that not all BRCA1 mutations are associated with biallelic BRCA1 loss in the tumor, thus communication is key for optimal interpretation of NGS results. The second key question is whether germline versus somatic mutations matters. Assessing outcomes from TOPARP-B, PROFOUND, and TRITON2, outcomes were comparable favoring PARP inhibitor treatment, regardless of germline versus somatic mutation assessment of BRCA1/2: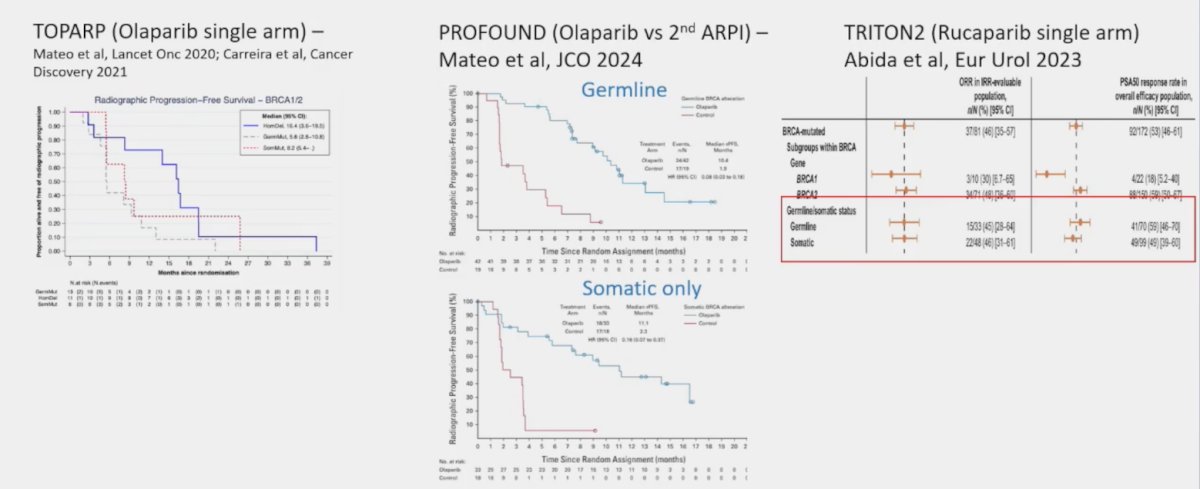
The third key question is when to treat, before or after taxane chemotherapy. Data from PROFOUND suggest that there was a radiographic progression-free survival benefit for both the taxane naïve and patients who had progressed on taxanes: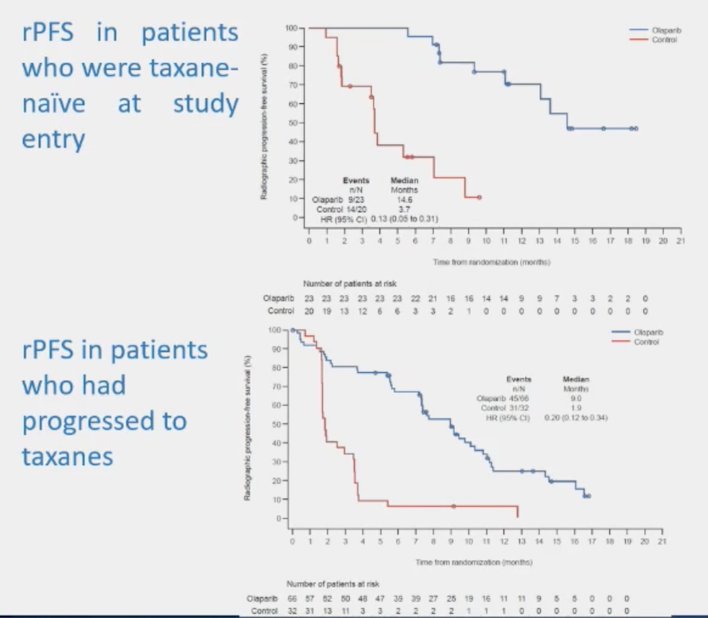
Similar outcomes were also noted in the TRITON3 trial of rucaparib. The fourth key question is what to do with combination therapy. We now have three trials in this disease space, including PROpel(abiraterone + olaparib),5 MAGNITUDE(abiraterone + niraparib),6 and TALAPRO-2(enzalutamide + talazoparib).7 Although there are variations in trial design and mutation inclusion criteria, generally there was a benefit for PARP inhibitor + ARPI versus placebo + ARPI: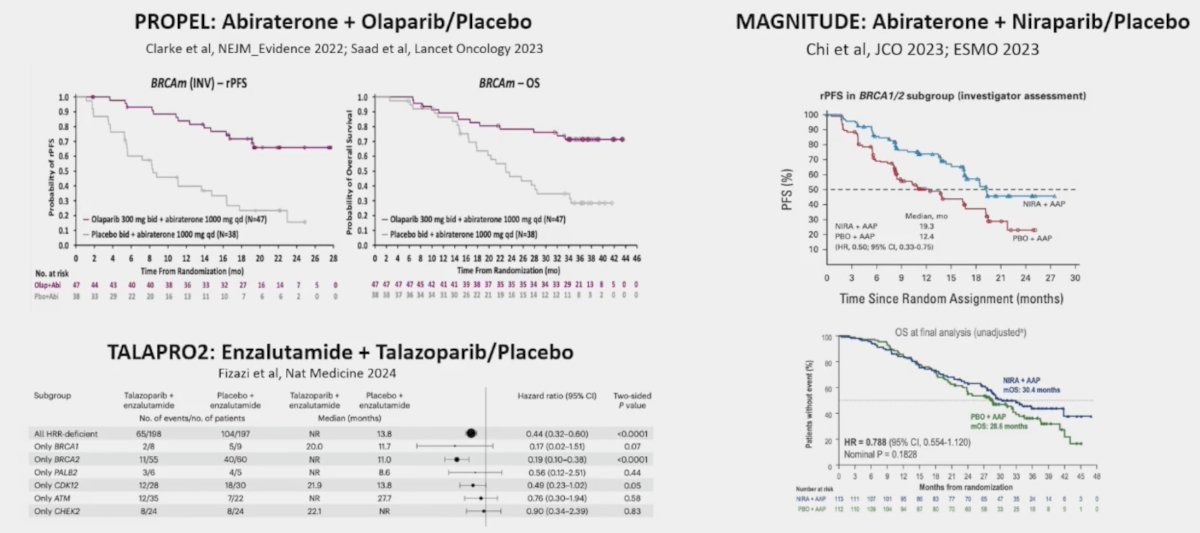
Dr. Mateo notes that combination trials suggest that PARP inhibition is better than no PARP inhibition for BRCA mutation mCRPC. However, there is a lack of data comparing combination versus sequential use. There is promising data from BRCAAWAY, but the caveat with this trial is the small sample size. Dr. Mateo’s view is as follows: ARPI + PARP inhibitor combinations are a reasonable option for ARPI naïve BRCA1/2 mutated mCRPC, acknowledging the lack of definitive data and risk of increased toxicities.
For mHSPC, ADT + ARPI +/- docetaxel +/- radiotherapy is standard of care. Notably, ARPI followed by ARPI switch has very limited efficacy and is discouraged in mCRPC by the guidelines. Why would this be any different in the mHSPC to mCRPC setting? In the 3 phase PARP inhibitor + ARPI combination trials, there are limited patients that had prior use of ARPI before the ARPI + PARP inhibitor trial treatment: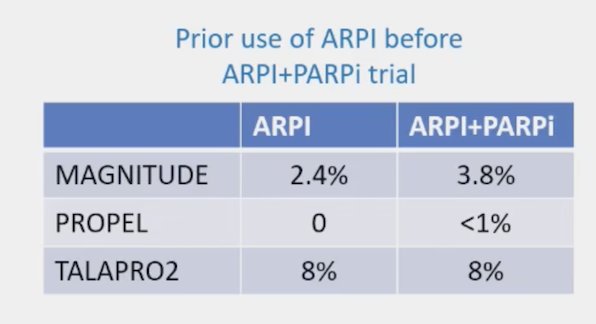
He emphasized again that PARP inhibitor monotherapy is approved post-ARPI in BRCA-mutated mCRPC. Dr. Mateo’s opinion is that we need to ask the relevant questions, noting that the following trial design would answer this relevant question: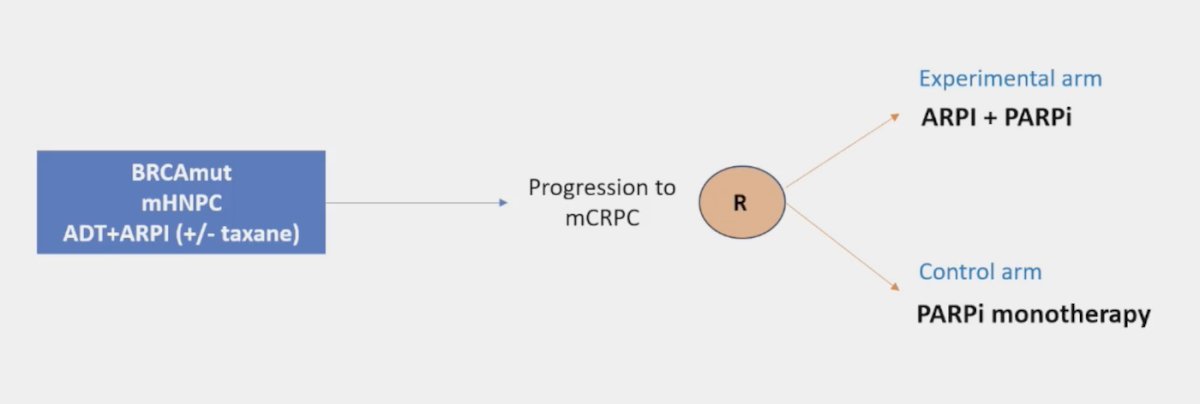
Dr. Mateo concluded his presentation with the following conclusions regarding PARP inhibitors for BRCA mutations:
- BRCA1/2 mutations associate with HRD in prostate cancer and are associated with a poor prognosis
- There is high sensitivity to PARP inhibitors, irrespective of the germline versus somatic origin of the mutation
- NGS testing should be considered in all m(CR)PC patients
- Trials confirm efficacy as monotherapy combination with ARPI in BRCA mutation patients. Thus, a PARP inhibitor is better than no PARP inhibitor
- There is no robust data comparing ARPI + PARP inhibitors versus ARPI followed by PARP inhibitors
- We need to generate that data through appropriate clinical trials
- In ARPI-naïve patients, the risk benefit ratio makes combinations acceptable until definitive data is available
- In the post-ARPI setting, the rationale for combinations is unclear, and PARP inhibitor monotherapy should be considered the standard option until proven otherwise
Presented by: Joaquin Mateo, MD, Vall d’Hebron Institute of Oncology, Barcelona, Spain
Written by: Zachary Klaassen, MD, MSc - Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Advanced Prostate Cancer Consensus Conference (APCCC) Meeting, Lugano, Switzerland, Thurs, Apr 25 - Sat, Apr 27, 2024.
Related content: The Use of PARP Inhibitors in mCRPC with BRCA1/2 Alterations - Joaquin Mateo
References:
- Mateo J, Porta N, Bianchini D, et al. Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): a multicentre, open-label, randomized, phase 2 trial. Lancet Oncol 2020 Jan;21(1):162-174.
- Mateo J, de Bono JS, Fizazi K, et al. Olaparib for the treatment of patients with metastatic-castration-resistant prostate cancer and alterations in BRCA1 and/or BRCA2 in the PROfound trial. J Clin Oncol. 2024 Feb 10;42(5):571-583.
- Fizazi K, Piulats JM, Reaume MN, et al. Rucaparib or Physician’s Choice in Metastatic Prostate Cancer. N Engl J Med. 2023 Feb 23;388(8):719-732.
- Abida W, Campbell D, Patnaik A, et al. Rucaparib for the Treatment of Metastatic Castration-resistant Prostate Cancer Associated with a DNA Damage Repair Gene Alteration: Final Results from the Phase 2 TRITON2 Study. Eur Urol. 2023 Sep;84(3):321-330.
- Saad F, Clarke NW, Oya M, et al. Olaparib plus abiraterone versus placebo plus abiraterone in metastatic castration-resistant prostate cancer (PROpel): final prespecified overall survival results of a randomized, double-blind, phase 3 trial. Lancet Oncol. 2023 Oct;24(10):1094-1108.
- Chi KN, Rathkopf D, Smith MR, et al. Niraparib and abiraterone acetate for metastatic castration-resistant prostate cancer. J Clin Oncol. 2023 Jun 20;41(18):3339-3351.
- Agarwal N, Azad AA, Carles J, et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): A randomized, placebo-controlled, phase 3 trial. Lancet. 2023 Jul 22;402(10398):291-303.
Related Content:


